Ennov Compliance platform
Ennov Workflow
Business Process Management Software
- Gain full control of your business processes
- Collaborate effectively with our 100% web-based solution
- Automate workflows to eliminate bottlenecks
- Standardize and eliminate variability
- Local language user interface connects global users
Advanced Process Management
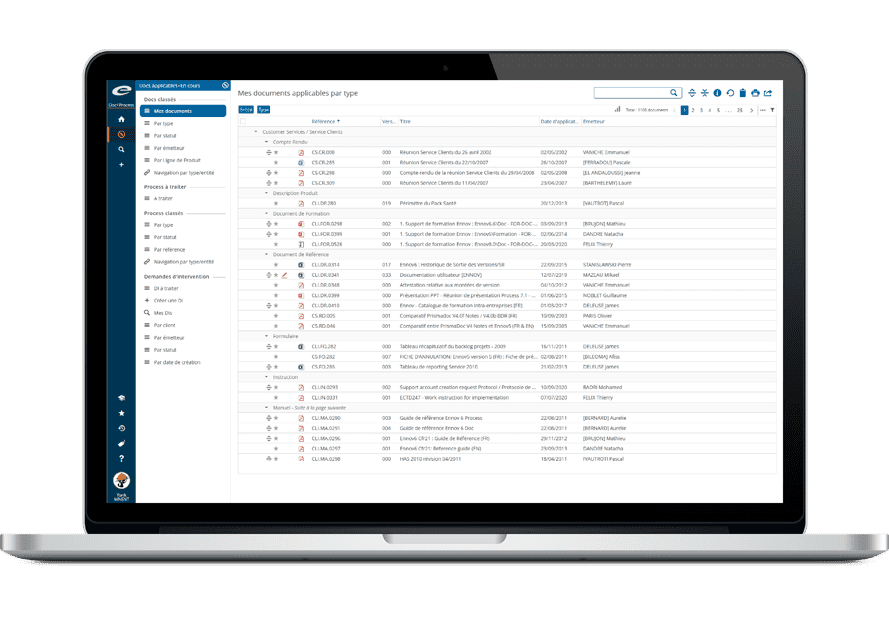
The Ennov platform includes its own workflow engine that provides advanced capabilities. Workflows are used in Ennov Training, Ennov eTMF and Ennov RIM. They allow control of the sequencing of tasks and data modification while providing visibility across the organization.
Workflows are entirely configurable by functional administrators (through a graphical interface). Coherence checks are performed whenever you save modifications in order to make sure they do not cause inconsistencies. Thus, Ennov guarantees workflows are coherent and reliable.
Highly Configurable and Dynamic Workflows
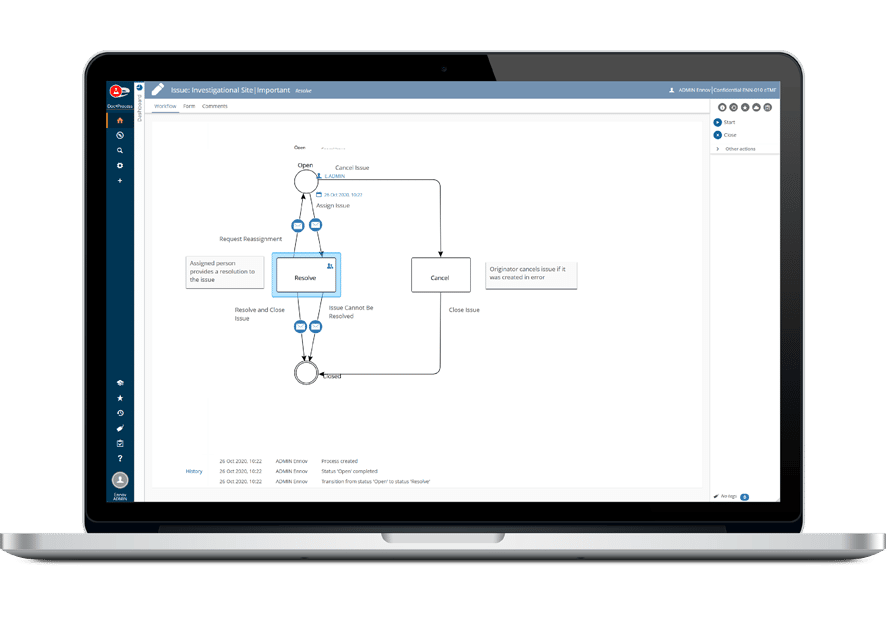
Each Ennov workflow is associated with a configurable form. Data entry is facilitated using dynamic sections that are displayed – or not – depending on previously entered field values (for example: the type of Incident). The connected user enters free text, selects values in picklists and adds additional files when needed. An electronic signature can also be required to complete a workflow step. If a field is editable at multiple steps, you can see the history of its values in the Ennov audit trail.
It is possible to create workflows that include several branches; depending on conditions set on metadata values, the workflow will be oriented one way or another. This makes it possible to cover different cases with a single workflow.
Ennov also offers the ability to initiate sub-processes: for example CAPAs launched from a Change Control, or Regulatory Activities created as part of an Activity Plan. Records that are linked together can exchange metadata (using inheritance mechanisms), which facilitates day-to-day work and eliminates the need for double entry. It is also possible to prevent the completion of the parent process as long as sub-processes are underway.
Escalation rules can be implemented if a workflow task or a process stays inactive for too long. In addition, Ennov includes a delegation feature so that processes are not blocked when a person is away from the office.
Facilitate Responsibilities and Deadlines
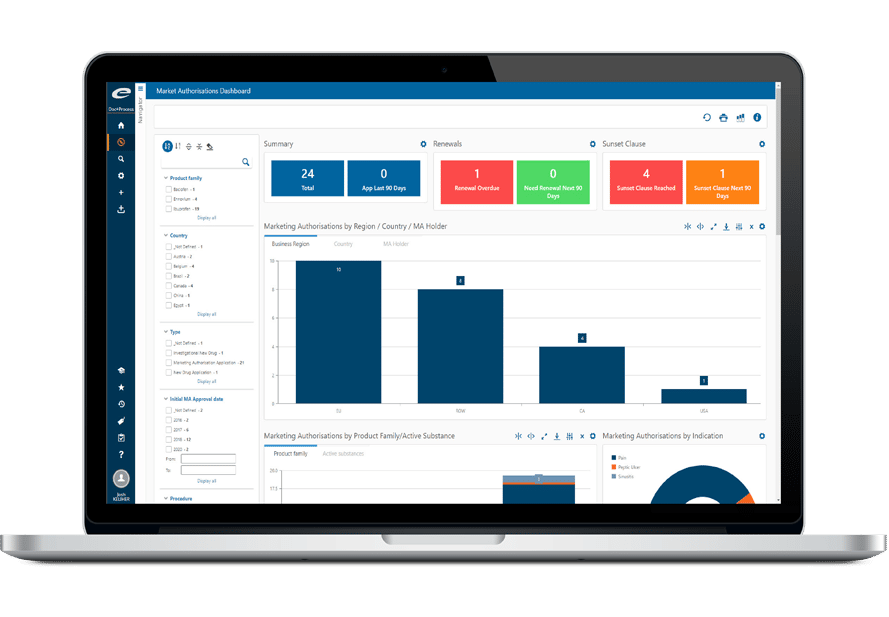
Responsibilities and deadlines are set in each workflow based on the management rules that are applicable in your organization. You can check at a glance who did what, if a task is late, if there is a bottleneck at some point of the processing, etc. Ennov offers powerful monitoring capabilities; managers see what percentage of workflows are completed, how many are initiated every month, what is the average processing time for a given step… Real-time KPIs are available by product, by country or by site, which provides a precious tool to evaluate and improve operational efficiency while planning future activities.
Ennov workflows can include automatic processing steps; the REST API also enables advanced integration to external systems.
Core Capabilities
- Graphical workflow designer
- Powerful workflow engine that supports sub-processes
- Visual forms editor
- "Smart" forms that adapt to process step and participant
- Manual or automated task execution
- Real-time monitoring with dashboards and statistics
- Full text and metadata based searching
- REST API for advanced integration
Key Features
- Integrated work list dashboard
- Configurable process metadata and views
- Automated email notifications
- Intuitive user interface
- Pre-configured for common quality processes
- Intrinsically connected to Ennov Doc and Ennov Dossier
- 21 CFR Part 11 Compliant
- 100% web-based
Ennov Platform
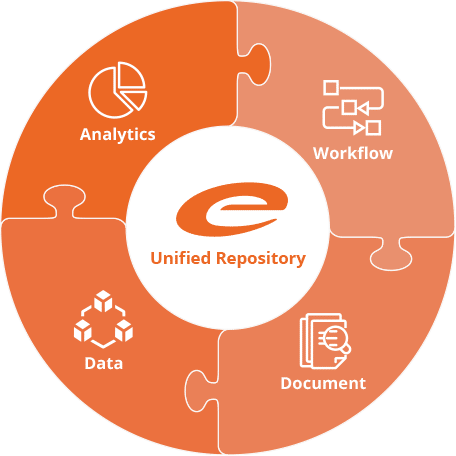
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
Why Choose Ennov ?
Hundreds of corporate customers trust Ennov
Over 20 Years of experience providing software solutions for Life Sciences
250+ life science customers, many more in other industries.
Modern architecture and interface
100% web-based. Highly scalable. User-centric design.
Our commitment to your success
Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Available as cloud-based or on-premises deployment
You can switch between deployment options at any time.
We make you autonomous
System configuration and management require no IT skills.
Improved security and optimized performance
Data is hosted locally for total flexibility. Single-tenancy minimizes business interruptions.

Cloud-based or On Premises

Multi-Platform