Ennov, Software Solutions for Life Sciences
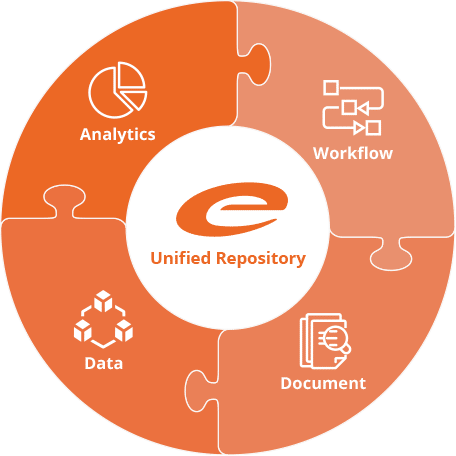
The only true unified platform and repository
to support and enrich the entire product life cycle.


Gain unlimited access to our library of exclusive content
Ennov
For more than 20 years, Ennov has been developing innovative, powerful and easy-to-use software for document and process management.
Ennov solutions are built on our Unified Compliance Platform which is designed specifically for the management of regulated content and processes.
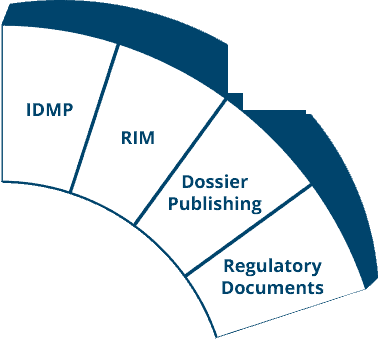
The Ennov platform is the technological foundation of our Regulatory (EDMS, Dossier Publishing, RIM, IDMP), Quality (EDMS, QMS) and Clinical (eTMF and CTMS) solutions.
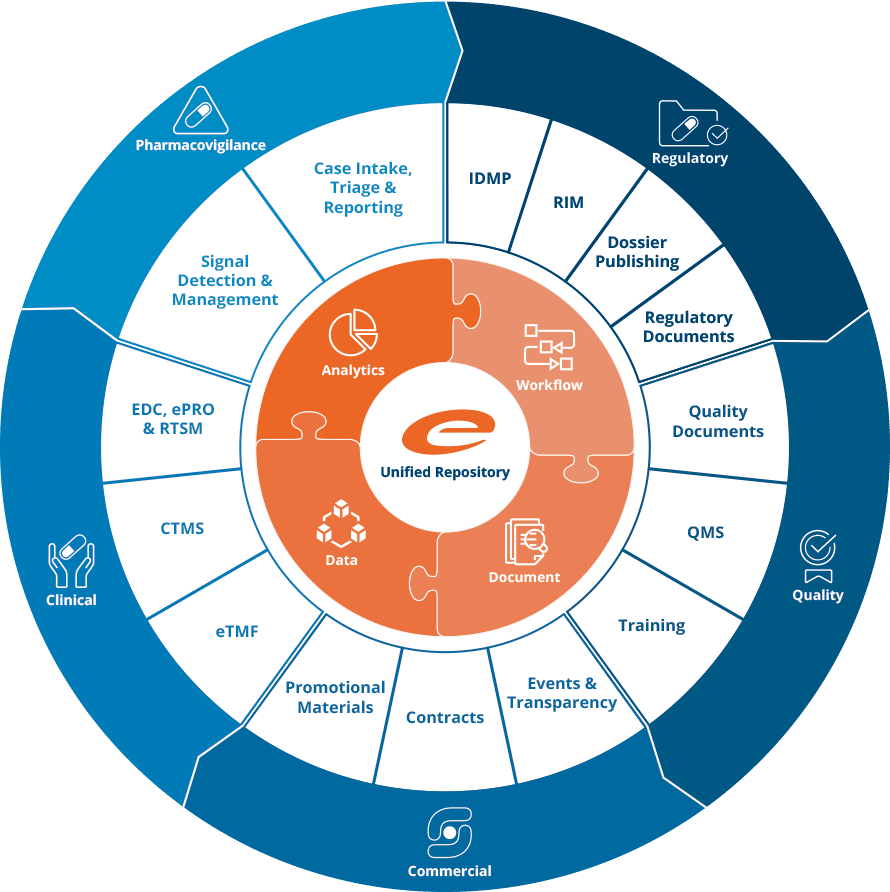
Ennov Platform
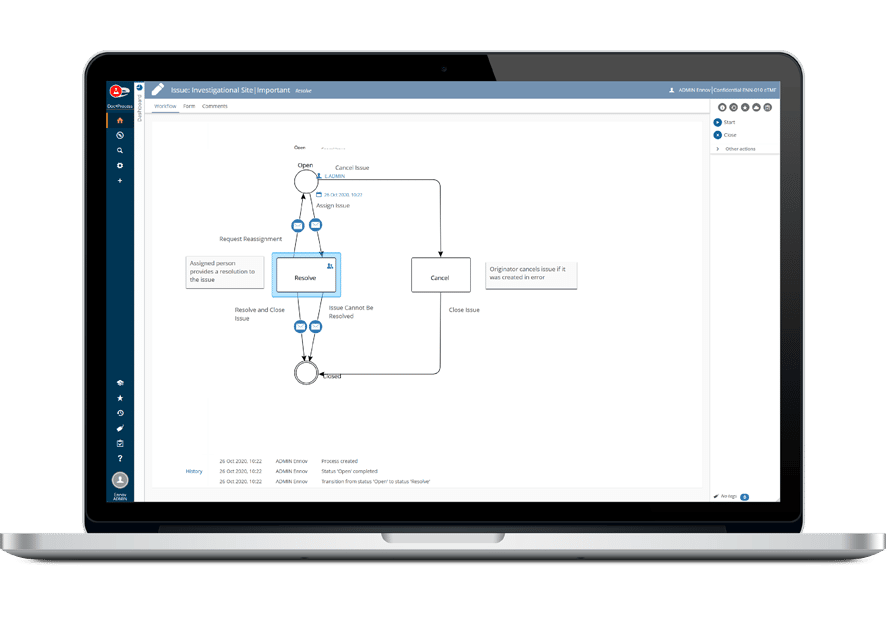
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
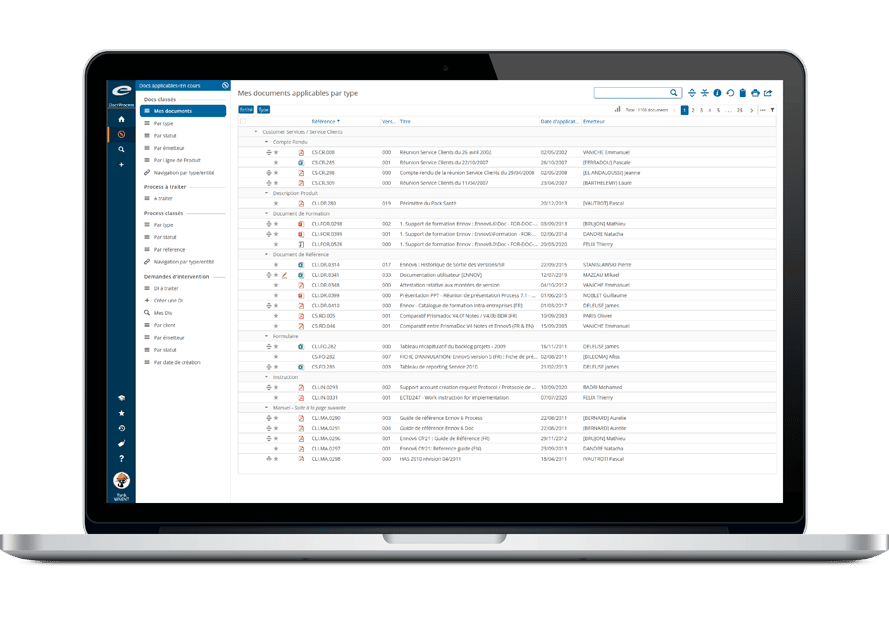
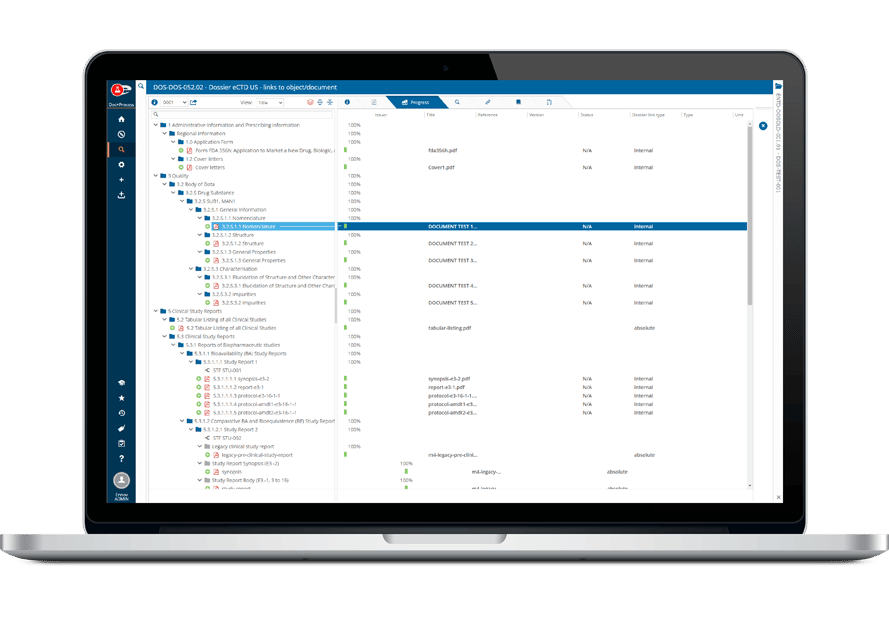
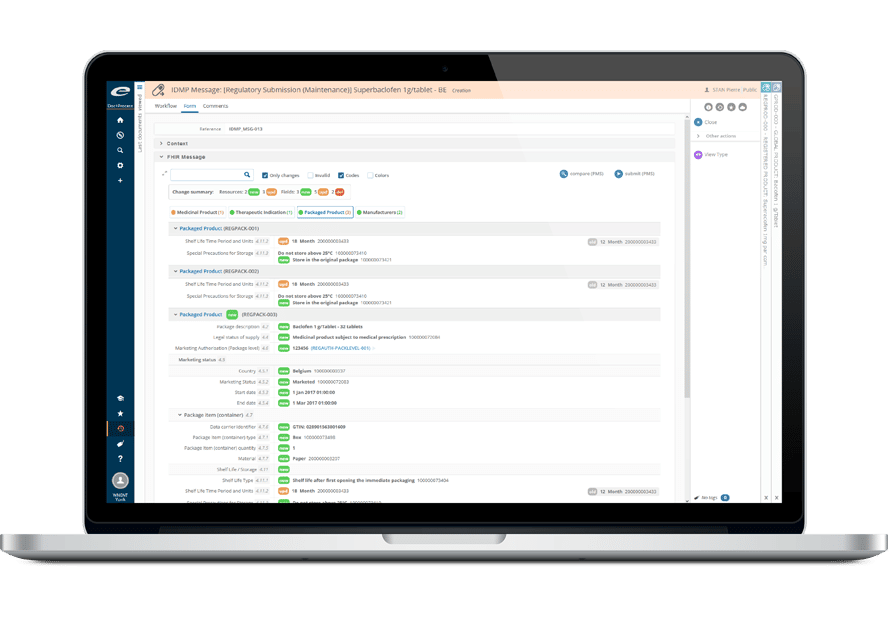
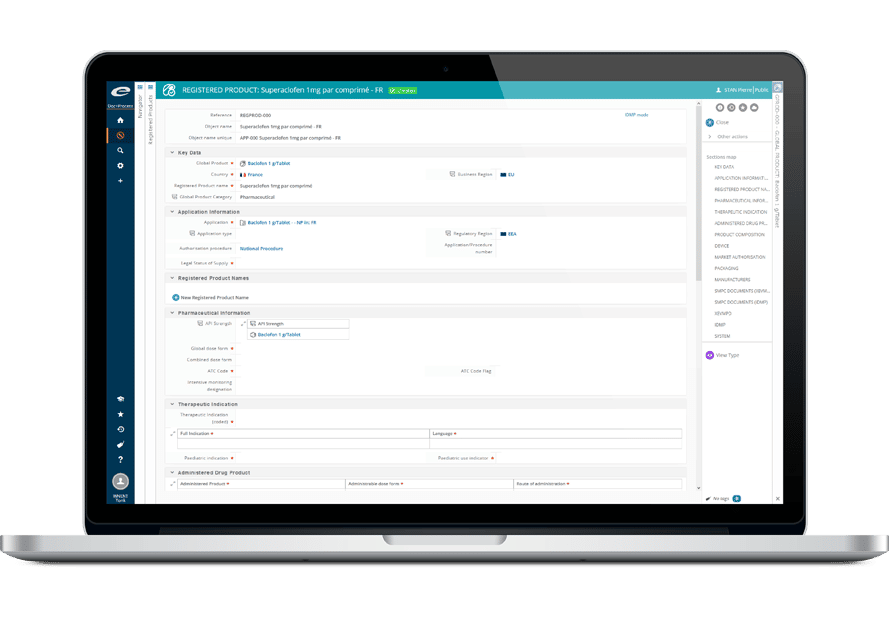
Ennov Regulatory
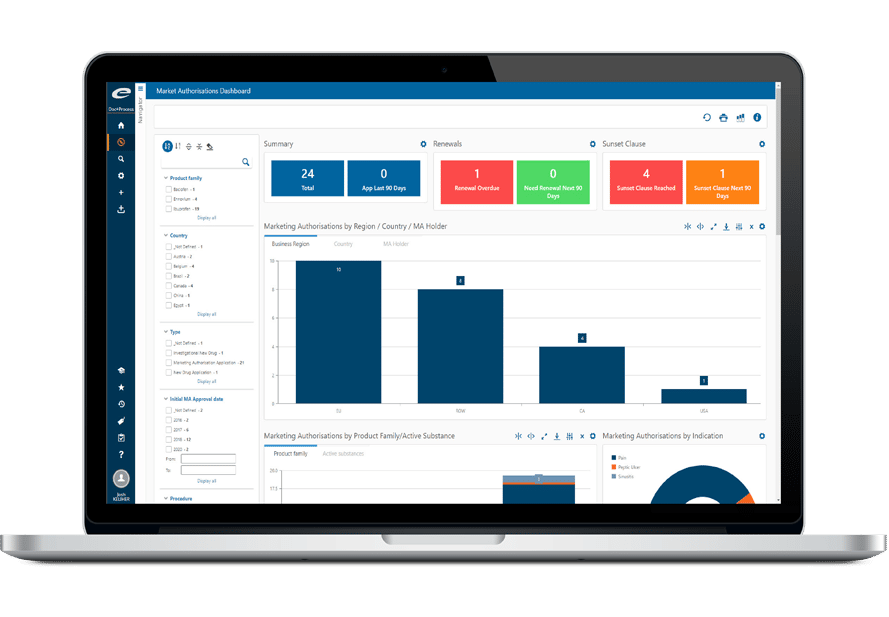
World-Class Regulatory Content And Information Management
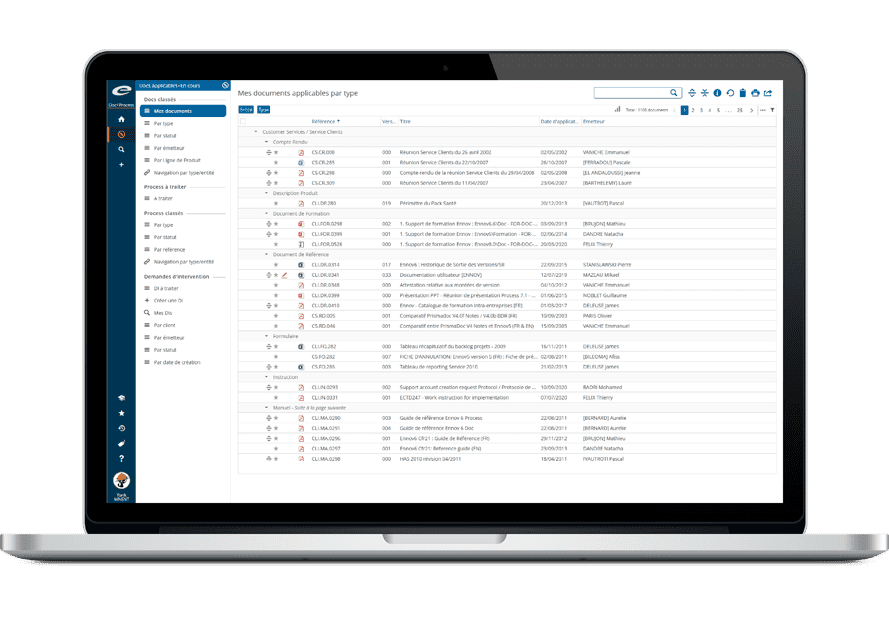
The Ennov Regulatory suite combines the power and flexibility of Ennov Doc, Ennov Dossier and Ennov Process to support the entire regulatory product lifecycle from the early planning of registration targets through product retirement. The Ennov Regulatory suite is an invaluable tool for regulatory activity planning, product registration management, dossier creation, dossier management and more.
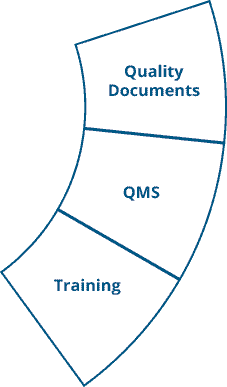
Ennov Quality
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best demonstrated practices. This allows Ennov Quality customers to get their system into production quickly and start realizing their return on investment. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
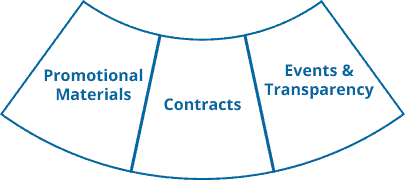
Ennov Commercial
Ennov Commercial is a comprehensive software solution for planning, organizing and monitoring all aspects of professional events including conferences, congresses, symposiums and customer communities while providing visibility and control over the logistics, budgets and regulatory compliance.
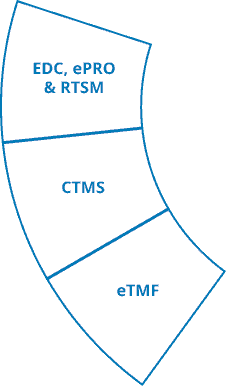
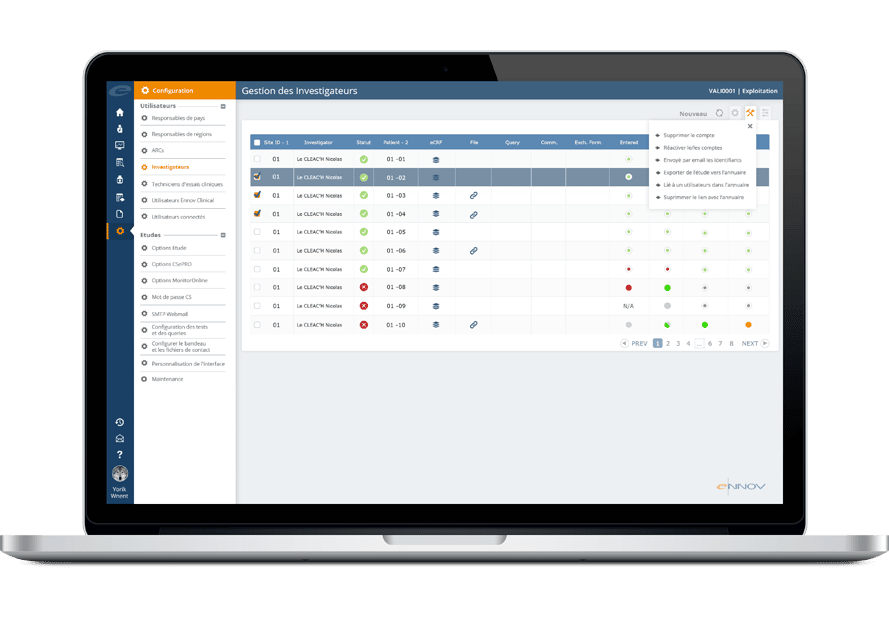
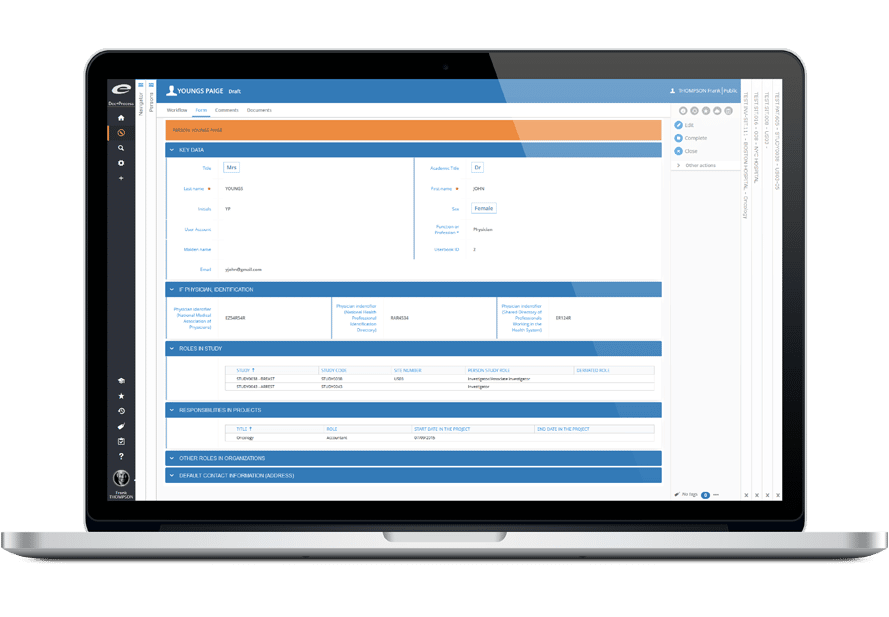
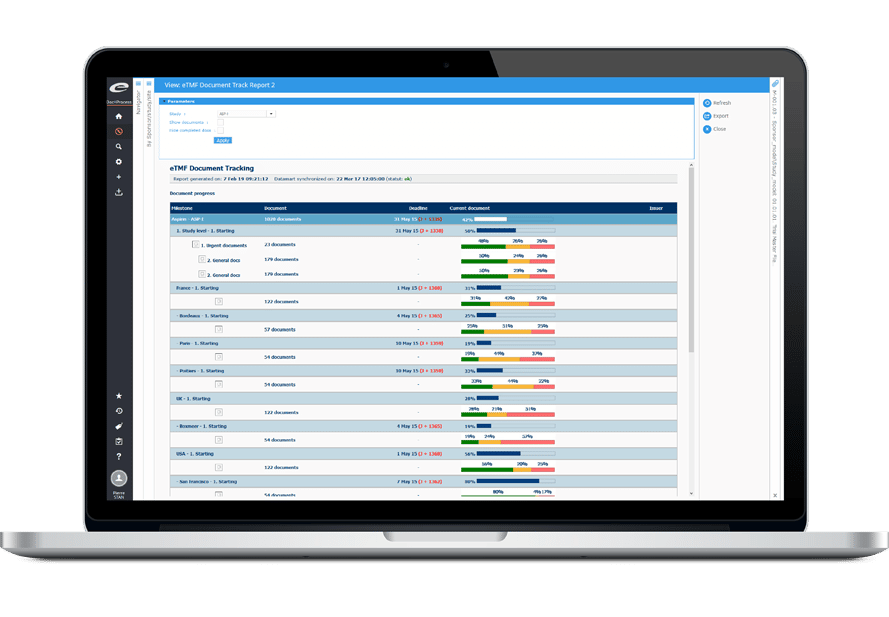
Ennov Clinical
The Ennov Clinical suite consists of Clinical Data Management applications (EDC, RTSM and ePRO) as well as Clinical Trial Management applications (CTMS and eTMF) that are available for deployment in the cloud or on premises.
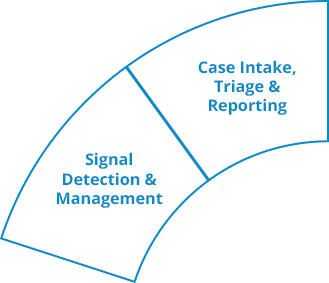
Ennov Pharmacovigilance
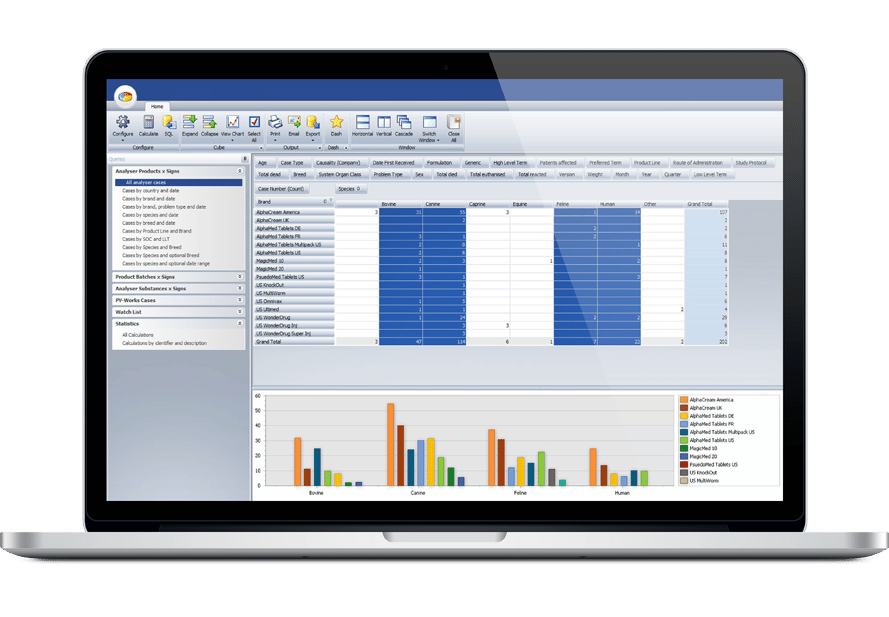
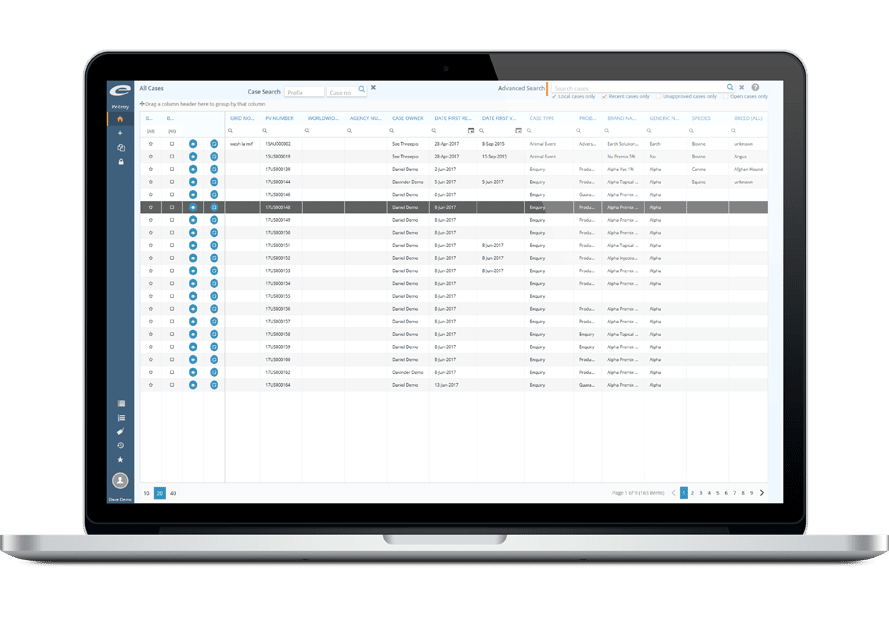
Ennov’s Pharmacovigilance suite keeps the collection, management, assessment, and reporting of human or veterinary adverse events in one unified database while also providing advanced signal detection and PV data analysis tools.
Become a Member of the Ennov Community
More than 300 Life Sciences companies around the world are powered by Ennov



Effective Regulatory Information Management can be complex. Your software shouldn’t be.
Discover how Ennov helped revolutionize their regulatory data management, saving valuable time and resources.
Resources
Take advantage of the knowledge and best practices gained from more than 20 years of research, innovation and development for the health and life sciences sectors.
Discover Innovative Software Solutions for Life
Providing a results-driven and customer-oriented experience
Customer satisfaction
Over 20 years of experience providing innovative, powerful and easy-to-use software for Life Sciences
Offering Our Customers a Full Compliment of Professional Services

Hosting
"Our flexible deployment options give you the freedom of choice"

Implementation
"Our optimized delivery methodology accelerates software implementation"
Training
"Ensuring our clients' success through targeted knowledge transfer"

Support
"When you need help we are just a phone call or click away"

Community
"Bringing our customers together for the benefit of all"
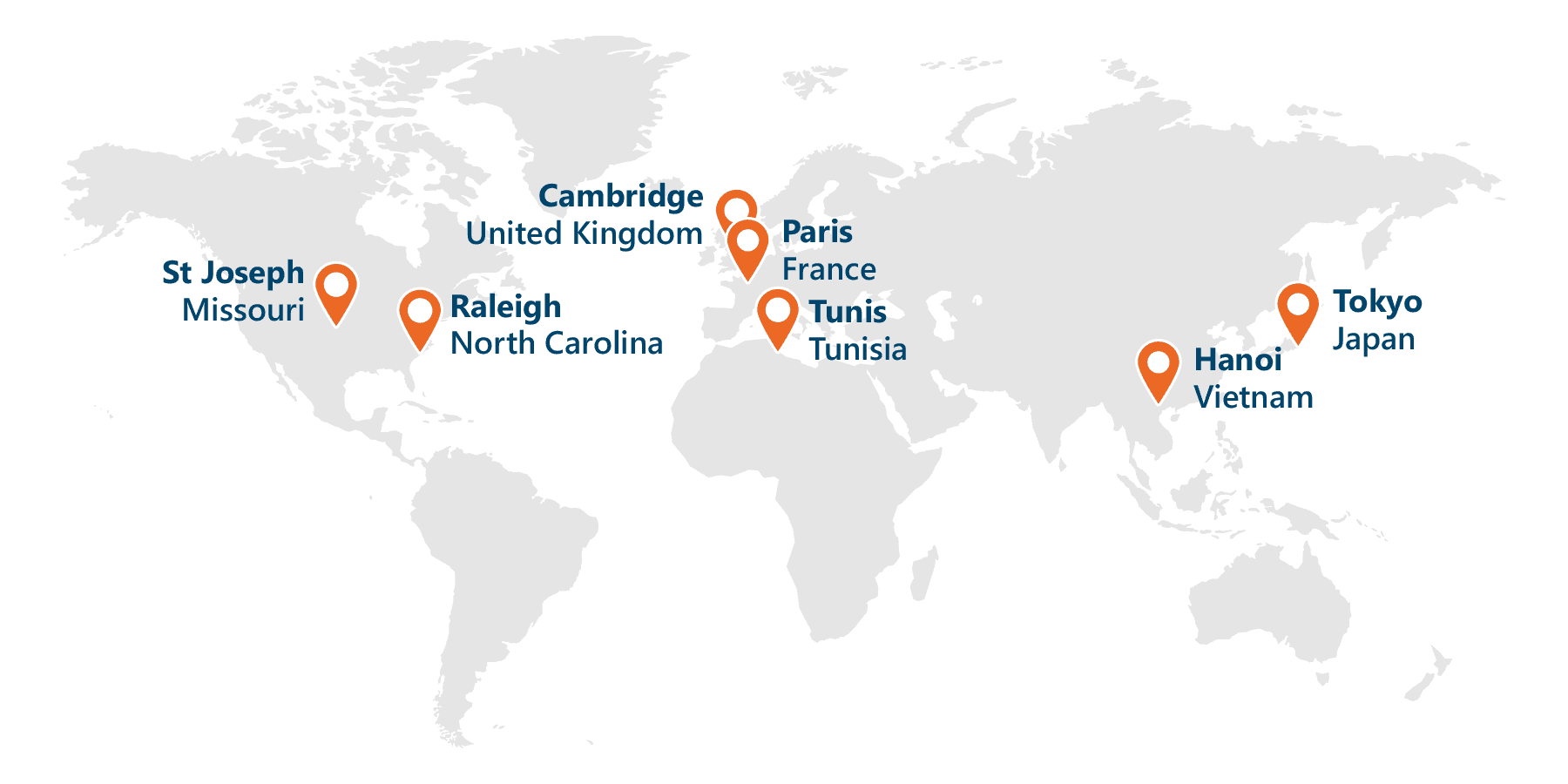
Service and Support on a Global Scale
Proudly serving over 250,000 users around the world