Unified Compliance Platform
A Unified Approach to Regulated Content and Data Management
Unified Access to All Documents for Improved Efficiency
Ennov’s unified compliance platform is built on a highly configurable software architecture. It combines a common, engaging user experience with powerful capabilities in business process management, document management, data and forms management, learning management, and business intelligence. These components form the foundation for all Ennov applications.
As a result, our applications are natively connected and do not require integrations or interfaces to facilitate interoperability. A common, harmonized and configurable data model eliminates redundancy and ensures data accuracy and consistency across all applications.
What Are the Benefits of the Unified Compliance Platform?
- Consistent UI for navigation, search, creation & tasks
- Common UX across all applications
- Lower training requirements and costs
- Lower total cost of ownership
- Unified master data management
- High user adoption rates
- Increased productivity
- AI is integrated in the platform, and is part of your workflows
Why A Truly Unified Platform Matters
Having a common, centralized and unified platform that is robust enough to support the diverse information management workflows from the earliest stages of research and development through market authorization is a common principle shared by companies striving to accelerate product release.
These companies also understand that adopting a unified compliance platform-based solution will help them drive harmonization, improve collaboration, ensure compliance, reduce costs and compete more effectively in global markets.
Ennov customers benefit from platform-wide updates that provide cross-application benefits with no additional costs. The Ennov compliance platform is 21 CFR part 11 compliant and open to integration with other enterprise applications through its robust REST API. Ennov is 100% web-based, highly scalable and highly secure.
Ennov Doc
Unified Access to All Documents for Improved Efficiency. Unified Management of Enterprise Content.
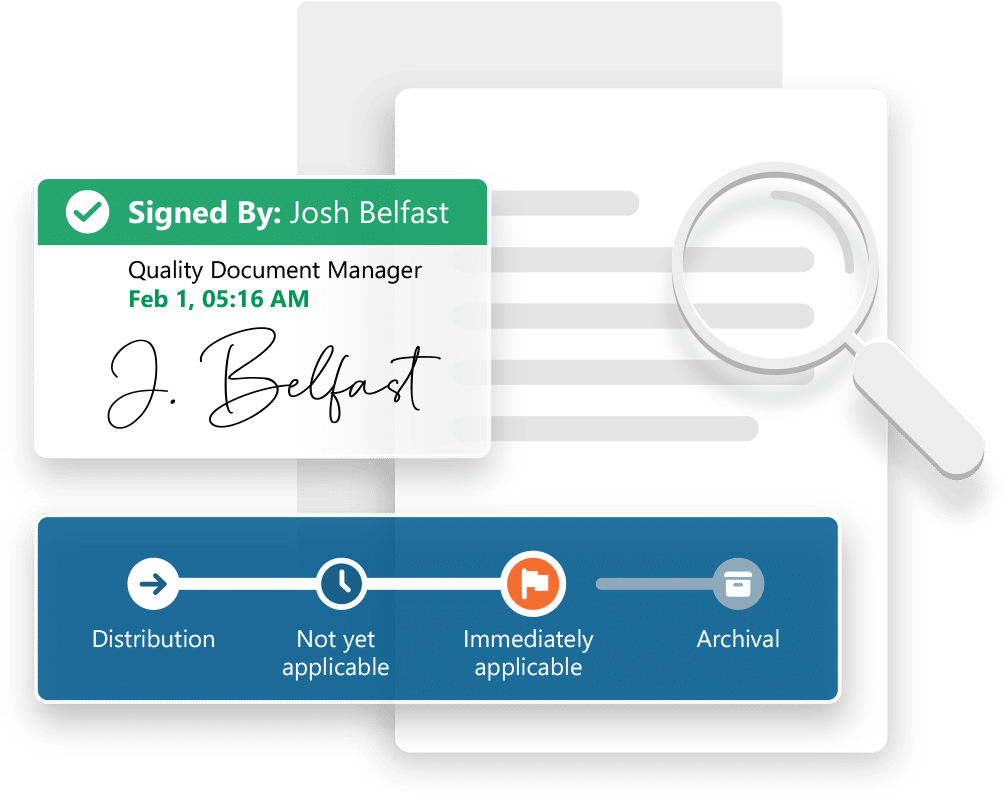
- Support for all document / content types
- Configurable workflows and electronic signatures
- Document control and version control
- Management of metadata in multiple formats
- Document rendition / dossier publishing
- Controlled document distribution
- Easy-to-use views and search features
Ennov Data
Robust Enterprise Data Management within a Single Repository.
- Data and metadata-based model
- Single data repository that spans traditional silos
- Monitoring of data consistency
- Fully integrated data change control process
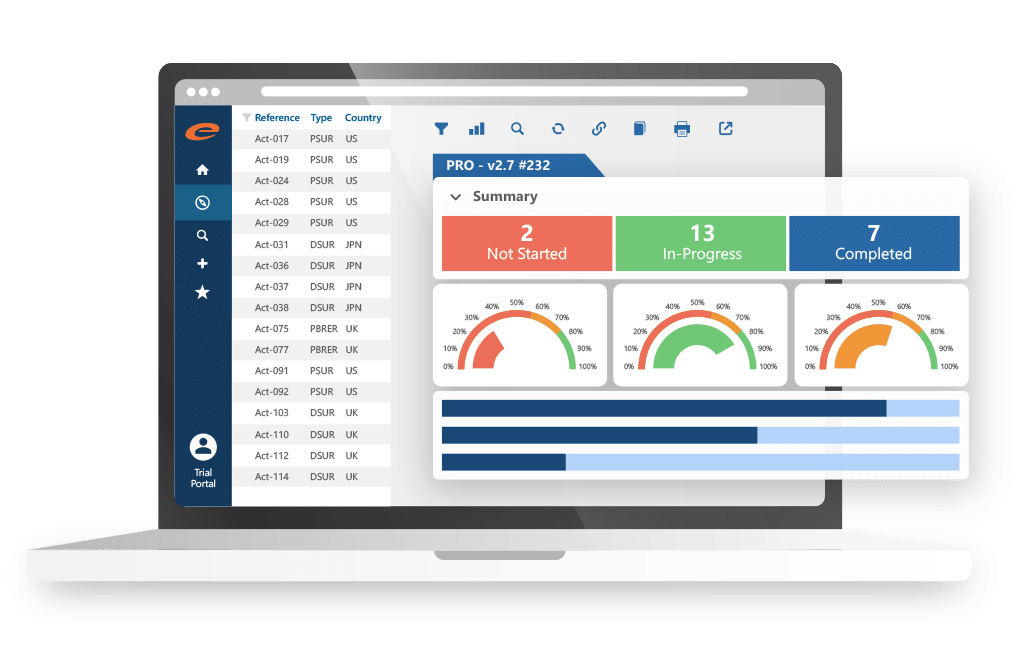
Ennov Workflow
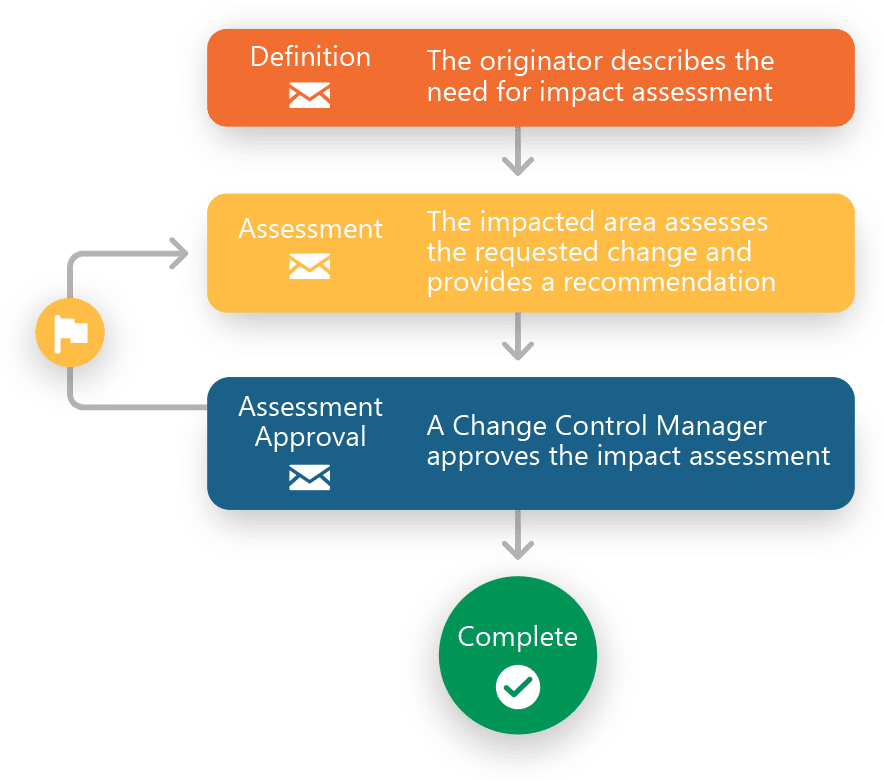
Manage and Track Workflows with Business Process Management Software.
- Automates business workflows to enforce SOP-defined sequences, roles, and rules
- Prevents stalled or off-track processes
- Fully configurable, user-friendly interface adapts to various organizational needs
- Email notifications and escalations prevent delays
- Enables tracking of tasks, activities, and participant progress to avoid bottlenecks
Ennov Analytics
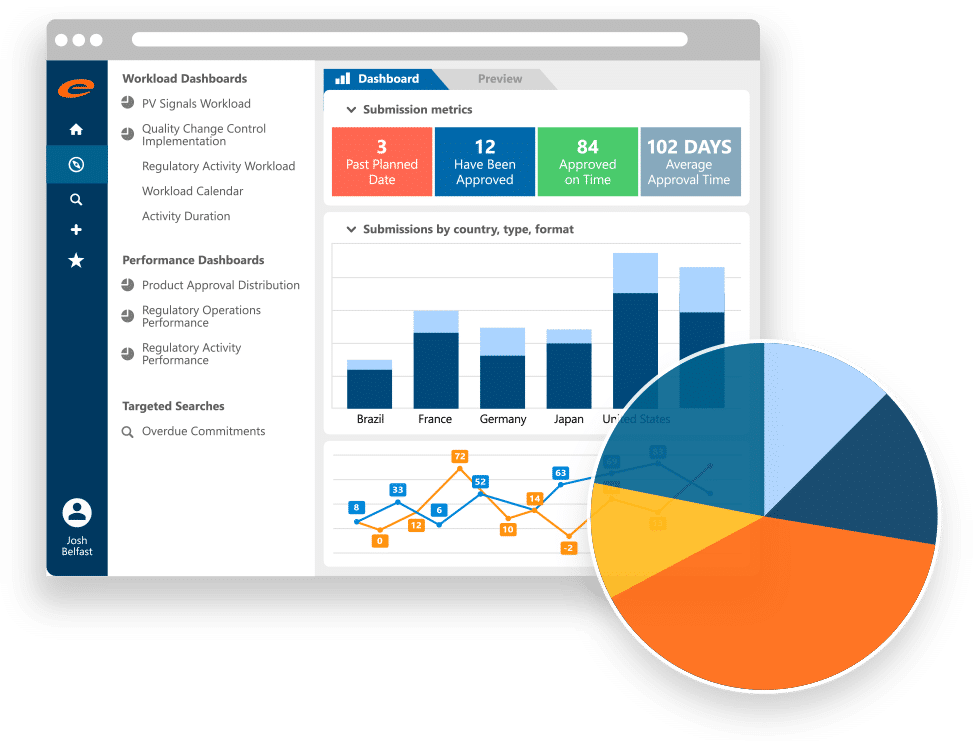
Optimize performance using our innovative Business Intelligence software.
- Provides powerful, user-friendly reporting and visualization tools
- Captures key metrics to generate KPIs from Ennov application data
- Native integration allows easy access via a common user interface
- Offers comprehensive access across all Ennov applications
- Includes rich visual display library with diverse charting options
- Enhance strategic decision-making with meaningful, actionable insights
Ennov AI
Advanced AI Capabilities. Integrated into our Unified Compliance Platform.
- Comprehensive NLP Frameworks
- Generative AI for Intelligent Solutions
- Machine Learning Capabilities
- Smart Document Classification
- Automated Document, Form, and Workflow Generation

Unified Platform Case Study
“The ability to manage global operations in one system, without multiple validations or constant vendor support, was transformational for our organization. What started as a quality initiative became our blueprint for enterprise-wide digital operations.”
IT Leadership,
Vetoquinol
