Ennov Quality Suite
A comprehensive QMS to improve efficiency and ensure compliance
Improves operational efficiency and ensure compliance with industry standards such as 21 CFR Part 11, GxP, Annex 11 and ISO. The Ennov Quality Core Model is comprised of a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best demonstrated practices. This allows Ennov Quality customers to get their system into production quickly and start realizing their return on investment.
Benefits
- A single authoritative source
Manage and track all quality related documentation, processes and data within a single application to streamline operations and ensure regulatory compliance. - Improved performance
Eliminate manual, paper-based processes and record keeping. Automate repetitive and error-prone tasks to achieve productivity gains.
- Global connectivity
Foster collaboration across organizational and geographic boundaries with a unified, harmonized solution that features an intuitive user interface with local language capabilities. - Increased visibility
Gain valuable insights through our AI-powered dashboard to identify and prevent problematic trends before they lead to quality issues.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
Pharmacosmos Case Study
“It makes our daily handling and signing of documents much faster, reduces the use of paper and eliminates signing documents by hand.”
Flemming Simonsen,
Director, GxP System Compliance Pharmacosmos
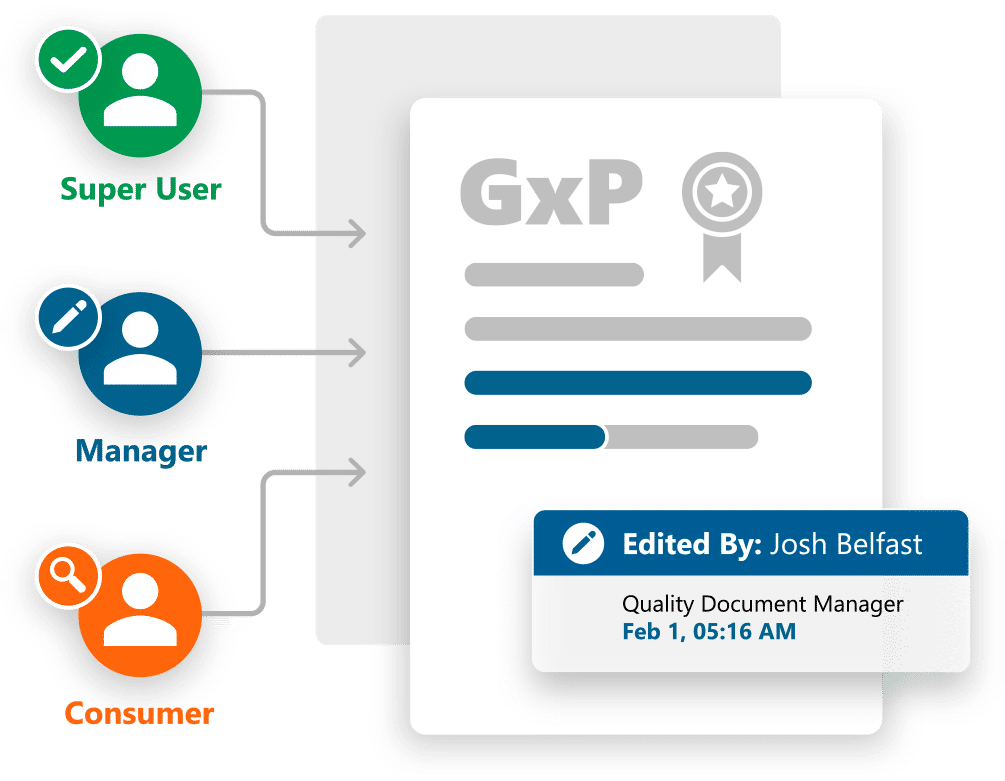
Ennov Doc for Quality
Manage and control GxP documents effectively and efficiently with Ennov Doc
- Predefined inventory of GxP documentation based on an industry standard reference model
- Full GxP document lifecycle support
- Collaborative authoring support via Microsoft Office Online integration
- Full support for Document Change Request, Periodic Review, and Controlled Copy processes
- Role-specific navigation for document Consumers, Contributors, Super Users and Supplier Managers
- Pre-configured views, reports and dashboards
Ennov QMS
Take control of GxP operations with Ennov Process
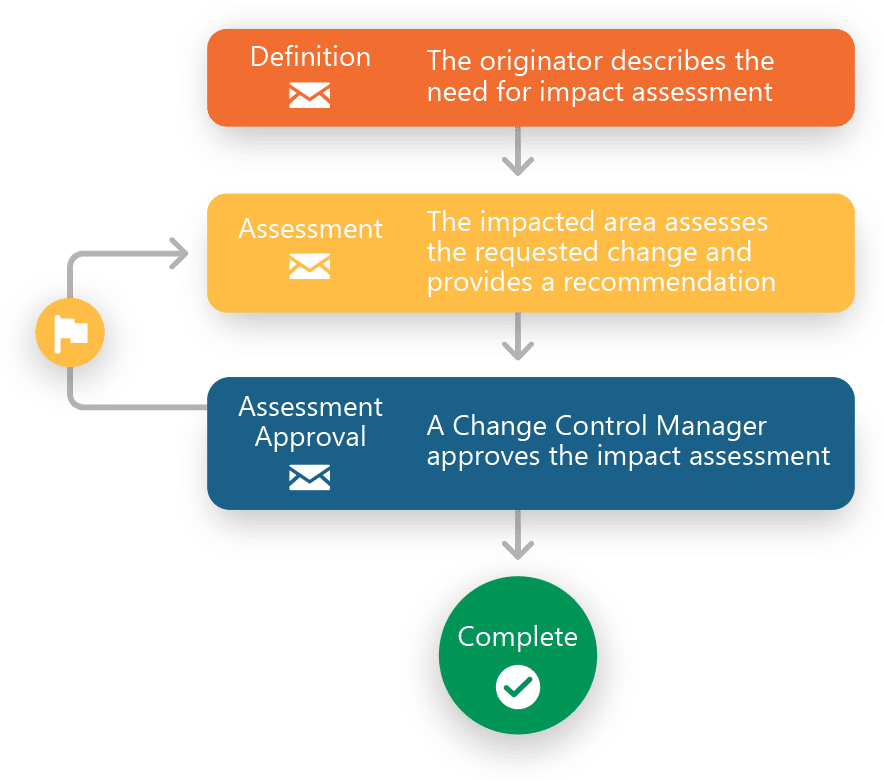
- Predefined inventory of processes including Deviations, Out of Specs, Complaints, Change Controls, Impact Assessments and CAPAs
- Comprehensive management of audit programs, audits and audit findings
- Supplier qualification ensures facilities, procedures and personnel comply with internal and Health Authority quality requirements and manages SCARs
- Role-specific navigation for Quality and Supplier Management personnel
- Pre-configured views, reports and dashboards
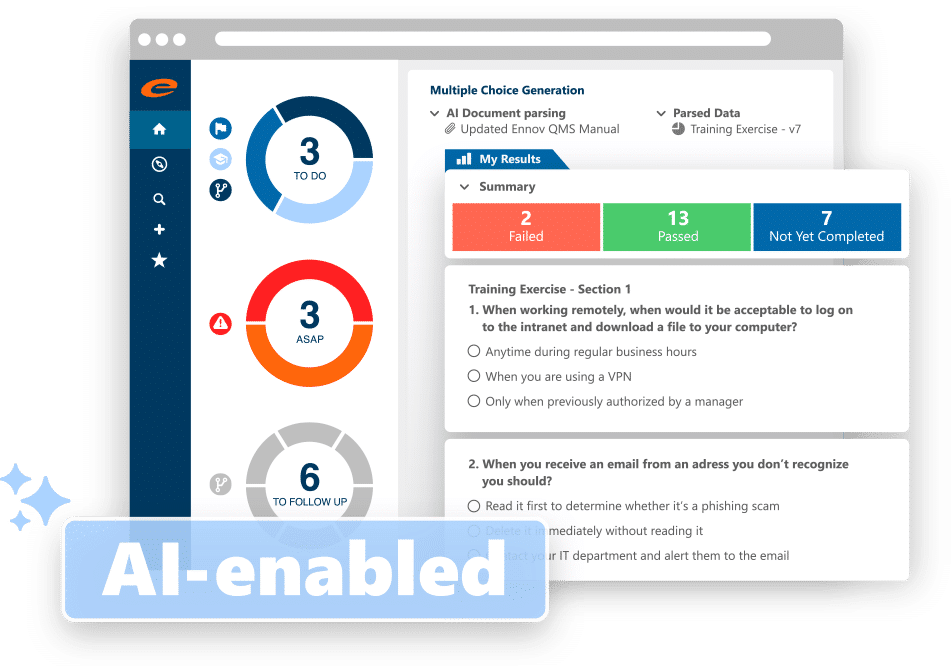
Ennov Training
Streamline GxP learning with Ennov Training
- Supports many different training methods including Classroom, On-the-Job, Multiple-Choice Questionnaire (MCQ), and Read and Understood (with verification)
- AI-enabled generation of MCQs
- Role-based curricula and qualification tracking
- Intrinsically integrated with Ennov Doc for Quality
- Robust tracking at the module, document, process, manager and trainee level
- Pre-configured views, reports and dashboards