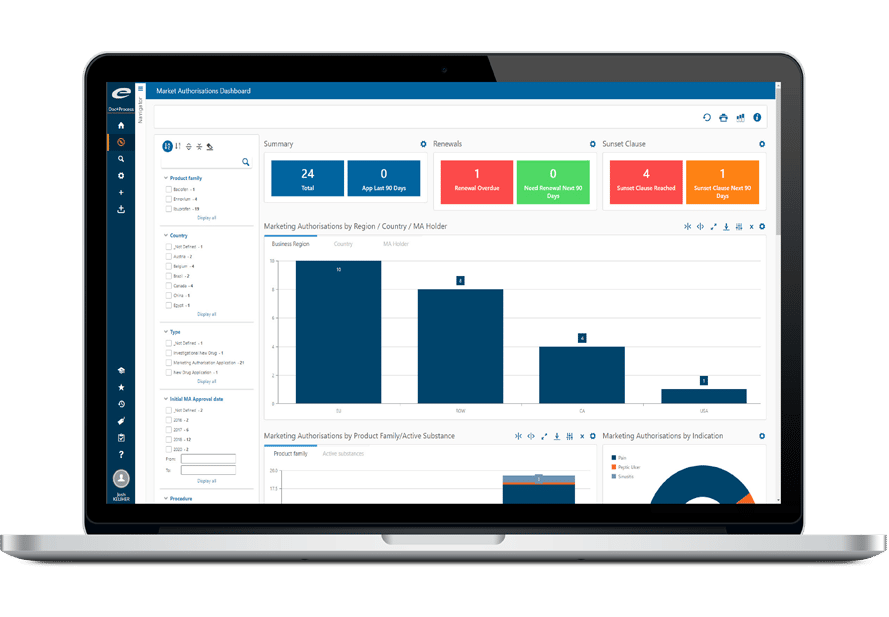
Ennov Compliance platform
Ennov Document
Enterprise Document Management Software
- Support of all documents types (including dossiers)
- Configurable workflows and electronic signatures
- Document control and version control
- Management of meta-data in multiple formats
- Document rendition / dossier publishing
- Controlled document distribution
- Easy-to-use views and search features
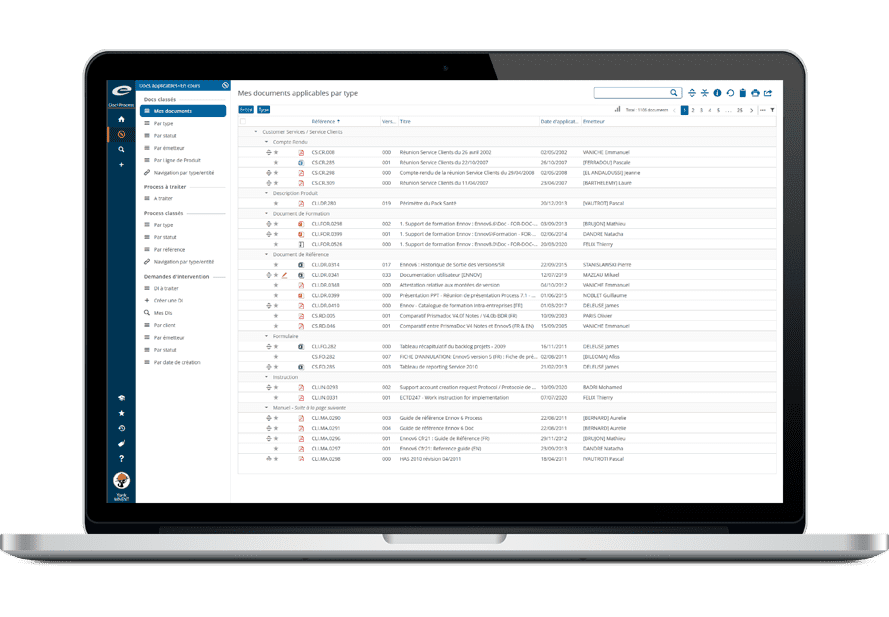
Unified Access to All Documents
All types of documents can be managed in the same Ennov database: Quality, Regulatory, but also R&D, Clinical, Engineering, Legal or Marketing. Each document has an ID form associated with it, it includes a set of metadata fields and displays the document history.
Field values are used to classify, filter and search documents. It is then very easy to structure the documentation and navigate in it; there is no need to create multiple databases, one for each domain. Document hierarchies can be set differently for each unit; users then access the content that is related to their function or interests.
Improved Productivity and Efficiency
Ennov manages the entire document lifecycle: creation, collaborative authoring, approval, distribution, revision/cancellation, archival. When a document is revised, the new version is created in draft status; it will become applicable after being signed by all approvers, the previous version is then automatically archived. Approval workflows are entirely configurable, they can be skipped in case you incorporate existing documents into the system. If large volumes of documents or dossiers have to be imported, Ennov proposes data migration services, this ensures a smooth transition to the new system.
Documents and dossiers managed in the Ennov EDMS can be linked to records managed in other modules (e.g. a Change Control in Ennov Process or a Regulatory Activity in Ennov RIM). Each view of documents has its own URL that can be inserted in an Intranet portal, for example.
A dedicated server converts documents into PDF format and dossiers into CTD, eCTD, NeeS, vNeeS and eCopy formats. Compliance with regulatory requirements is then guaranteed. Print control features can be activated if needed for specific document types such as batch files.
Document management is a key component of the Ennov platform.
Core Capabilities
- Manage any document format
- Advanced life cycle management
- Flexible rights management
- Automatic PDF rendering and tag management
- Full text and metadata based searching
- Controlled printing, copy and paste
- Periodic review, expiration and archive management
- Office 365 and Google Drive connectivity
- Complete PDF publishing solution
- Publication life cycle management
- Automatic table of contents generation
- Robust hyperlinking and bookmarking
- Composite document support
- Ability to define publication templates
Key Features
- Integrated work list dashboard
- Configurable document types, workflows and views
- Automated email notifications
- Integrated PDF Viewer
- Composite document support
- 21 CFR Part 11 compliant
- 100% web-based
- Intuitive drag-and-drop user interface
- Compatible with any WebDAV compliant repository
- Automatic compliant PDF rendering
Ennov Platform
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
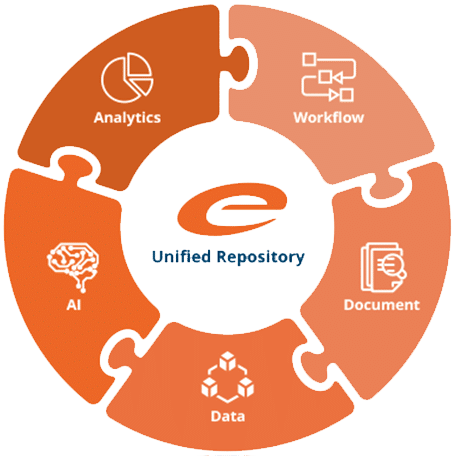
Ennov Platform
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
Why Choose Ennov ?
Over 500,000 users trust Ennov
Over 25 years of experience providing software solutions for Life Sciences
450+ Life Science customers, many more in other industries.
Modern architecture and interface
100% web-based. Highly scalable. User-centric design.
Our commitment to your success
Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Available as cloud-based or on-premises deployment
You can switch between deployment options at any time.
We make you autonomous
System configuration and management require no IT skills.
Improved security and optimized performance
Data is hosted locally for total flexibility. Single-tenancy minimizes business interruptions.

Cloud-based or On Premises

Multi-Platform