Ennov Dossier
Accelerate approvals with our intuitive and compliant Submission Publishing software
- Create, manage and publish Regulatory submissions
- Publish to any output format
- Robust hyperlinking and bookmarking
- Built in validator ensures compliant submissions
- 100% web-based, ideal for global deployments
The Submission Publishing Challenge
Authoring, reviewing, approving and tracking documents that are destined for global regulatory submissions can be a challenge. Submission teams across multiple departments and locations must collaborate effectively to ensure that timelines and agency commitments are met.
Storing documents on file shares across disparate locations and emailing them among team members is inefficient, impedes productivity and introduces risk. Regulatory Operations staff often needlessly spend time hunting for the correct version of documents – prolonging their tasks at hand and increasing the possibility of missed deadlines.
If these challenges sound all too familiar, our comprehensive full-featured Enterprise Document Management System, Ennov Doc for Regulatory can help you streamline document management processes and increase your operational efficiency.
Efficient and Easy to Use
Ennov Dossier provides the ability to build, manage, publish, validate and archive regulatory dossiers using the native capabilities found within Ennov Doc.
This eliminates the fragmented and inefficient processes of locating, copying and uploading the documents that you need for your regulatory submissions – providing a harmonized and seamless dossier publishing solution.
A simple drag-and-drop interface allows publishers to link documents into submission assemblies quickly and easily.
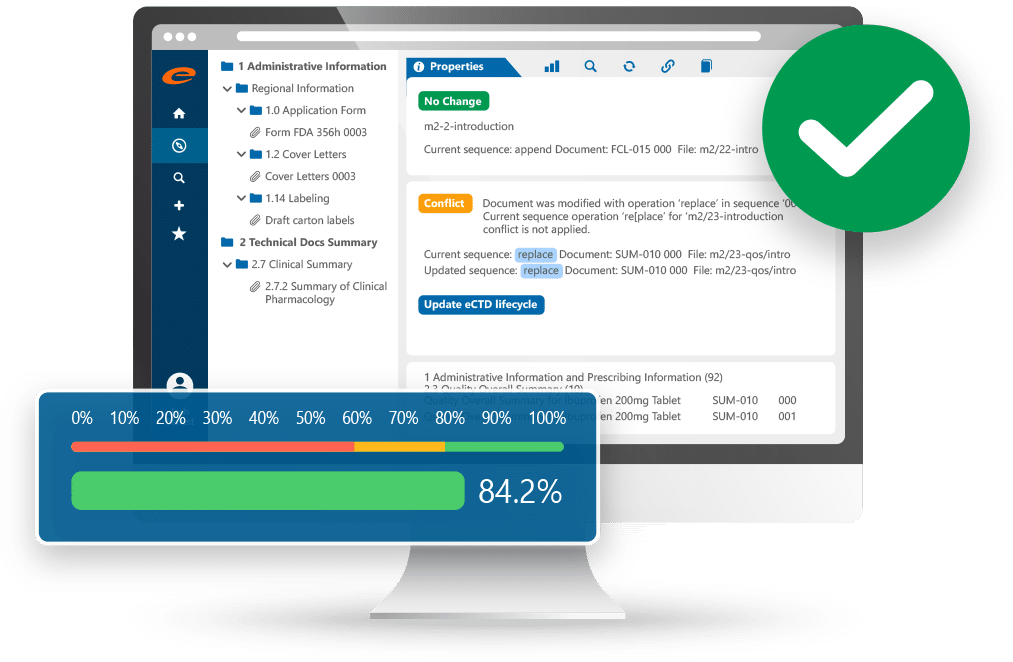
Improve Productivity with our Integrated EDMS
Ennov Dossier, when combined with Ennov Doc, provides the ability to manage your regulatory dossiers using all of the robust functionality of our comprehensive EDMS.
Automated dossier life cycles, workflows and notifications eliminate fragmented manual processes and increase productivity.
Ennov’s unique metadata-based navigation also helps improve productivity by allowing users to quickly locate dossiers using their properties rather than a storage location in a filing hierarchy.
Compliant Submissions Every Time
Submission assembly templates are provided for the regions that accept eCTD submissions (e.g. US, EU, GCC, Canada, Swissmedic, TGA) as well as for other non-eCTD formats and can be modified to meet a client’s specific requirements. Ennov provides regular updates to these templates as the regulatory guidance changes.
Ennov Dossier also provides the ability to create Tables of Contents, hyperlinks, bookmarks and other navigation aides to assist in the review of the dossier.
During publishing all the necessary components for a compliant submission, including the required ICH and regional XML files, correctly named leaf files and folder structures are created.
Core Capabilities
- CTD, eCTD, NeeS, VNeeS and eCopy support
- Dossier life cycle management
- eCTD sequence and metadata management
- Robust hyperlinking and bookmarking
- Integrated eCTD validator
- Built-in submission assembly templates
- Full text and metadata based searching
Key Features
- Intrinsically connected with Ennov Doc
- Intuitive drag-and-drop user interface
- Compatible with any WebDAV compliant repository
- Automatic compliant PDF rendering
- 100% web-based
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
