Ennov Pharmacovigilance Suite
An end-to-end solution for collecting, reporting and analyzing human and vet PV data
Used by pharma companies, CROs, and health authorities around the world, Ennov’s Pharmacovigilance suite brings everything together (from case intake to signal detection) in one unified system. You can collect, manage, assess, and report human and veterinary adverse events in one seamless workflow — with built-in tools for advanced analysis and smarter decisions.
Benefits
- A single authoritative source
Manage and track all pharmacovigilance data within a single, unified database to streamline PV operations and ensure regulatory compliance. - Improved performance
Eliminate manual, paper-based processes and record keeping. Automate PV case intake and reporting to achieve productivity gains.
- Ensure compliance
Gain total confidence that all aspects of achieving and maintaining regulatory compliance, including electronic reporting, will be met. - Increased visibility
Get a real-time overview of your safety database with dynamic metrics and dashboards.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
PDS Case Study
“We were happy to find the support and respect Ennov demonstrated, which we felt was quite rare in a shared business partnership.”
Phil Turner,
Business Development Manager Pure Drug Safety
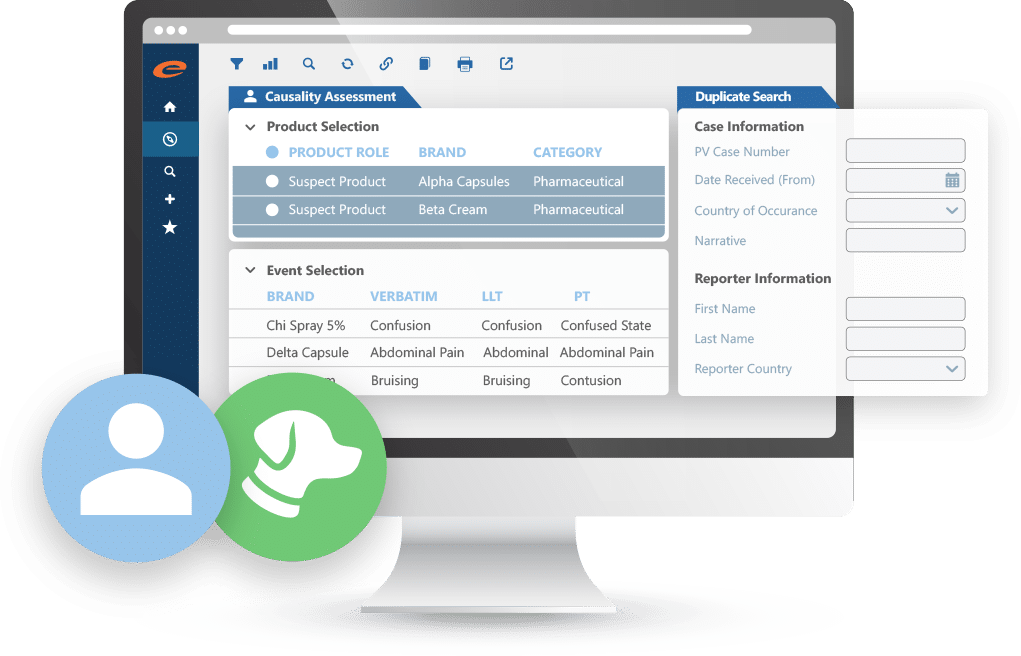
Case Intake & Management
Capture confidently. Configurable workflows. Stay compliant.
Efficiently manage human and animal pharmacovigilance case data from any source (clinical trial or post-marketing) with Ennov’s flexible, compliant solution. Import reports in various formats or enter them directly using fast, intuitive templates. Configurable workflows keep cases on track from intake through coding, assessment, and reporting.
Why teams choose Ennov for case intake and management:
- Accurately capture and manage both human and veterinary PV cases
- Import adverse event data or enter directly using flexible templates
- Fully configurable workflows to support end-to-end case processing
- Seamlessly integrates into existing pharmacovigilance operations
- Trusted by pharma, CROs, service providers, and regulators
- Scalable, compliant, and designed to fit your business processes
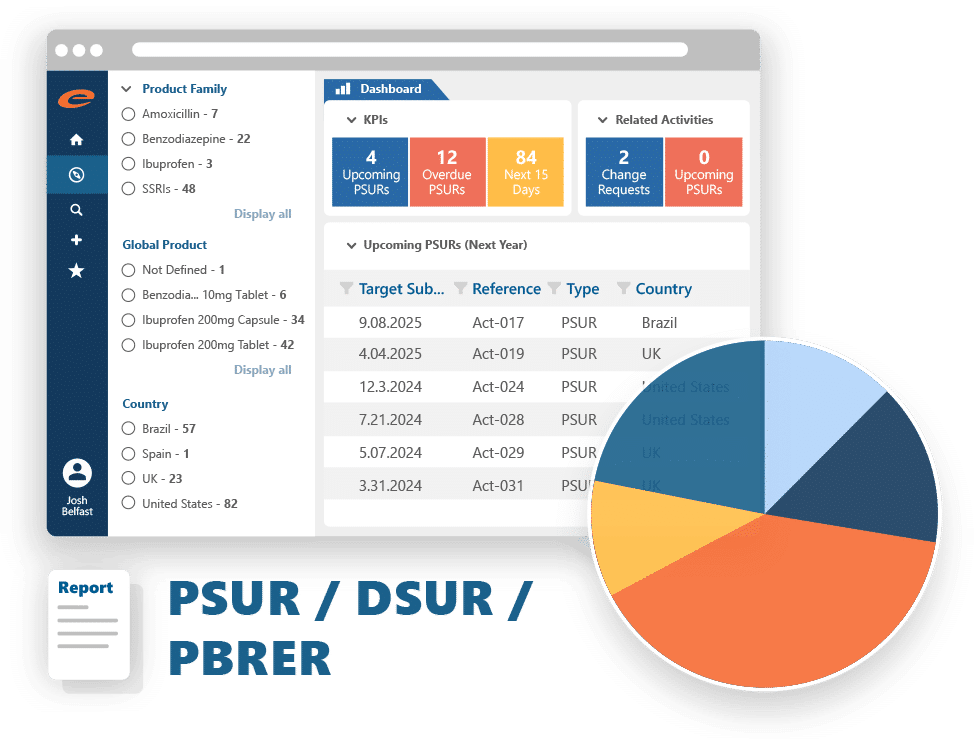
Reporting & Data Analysis
Compliant by default. Fast by design. Global by nature.
A compliant, easy-to-use pharmacovigilance tool giving you flexibility, automation, and confidence — without the typical complexity of reporting.
Why teams choose Ennov for compliant reporting and analysis:
- Generate data extracts, paper reports, and XML submissions with ease
- Analyze safety data using pre-supplied or custom-built queries
- Seamlessly submit to global health authorities including FDA and EMA
- Integrated E2B-compliant gateway streamlines electronic submissions
- Supports all standard single case and aggregate reports
- Offers both manual and automated generation and submission options
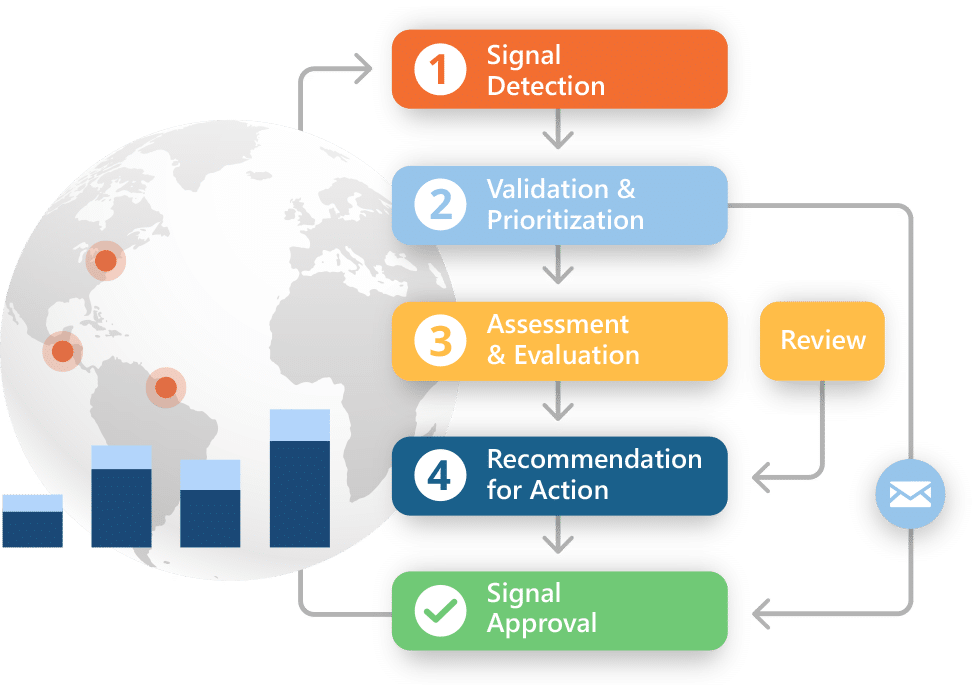
Signal Detection & Management
Spot the signal. See the trend. Stay ahead.
Intuitive analysis helps you identify trends, track signal activity over time, and stay aligned with internal risk processes. All without complex setup or customization.
Why teams choose Ennov for signal detection and management:
- Out-of-the-box signal detection and data mining for business users
- Access to advanced statistics, data cubing, and visualizations
- Calculate PRR, ROR, and MGPS to assess safety signals
- Apply smart stratification to surface threshold-based trends
- Searchable repository to track and review signal evolution
- Configurable workflows aligned with internal risk processes