Ennov CTMS
Clinical Trial Management Software
One system for trial oversight, site management, and operational control.
- Comprehensive CTMS software
- Integrated with Ennov Clinical Data Management
- Clear visibility into investigational sites
- Integrated workflow
- 100% web-based
The Clinical Trial Management Challenge
Each year the Life Sciences companies look for new ways to improve the efficiency and reduce the costs associated with running clinical trials. However, there are many obstacles that can prevent them from achieving their goal. Probably the most significant obstacle they must overcome is the effective selection, monitoring and management of investigational sites.
These companies recognize that site efficiency and productivity can be improved by centralizing information, providing recruitment and screening tools, automating scheduling, managing finances, and providing accurate reporting and metrics. These challenges can be met using Clinical Trial Management software (CTMS), which serves as a single authoritative source for operational data related to the planning, management and reporting of clinical trials.
A Single Authoritative Source for Clinical Trial Information
Ennov CTMS facilitates the end-to-end management of clinical trials. The software allows sponsors to be more efficient, to make better decisions, to ensure compliance, to select investigators well, to monitor patient recruitment and to manage finances. Ennov CTMS includes comprehensive directories to manage site personnel (investigators, sub-investigators, study coordinators, pharmacists) and organizations (hospitals, competent authorities, CROs, suppliers).
Ennov CTMS provides functions for tracking patients, patient visits, EDC data, activities, queries, deviations, adverse events, drug supply, monitoring visits and finances. A full complement of reports and dashboards provide total visibility into the status of each study, country, site and investigator to provide the information necessary to make the best decisions possible.
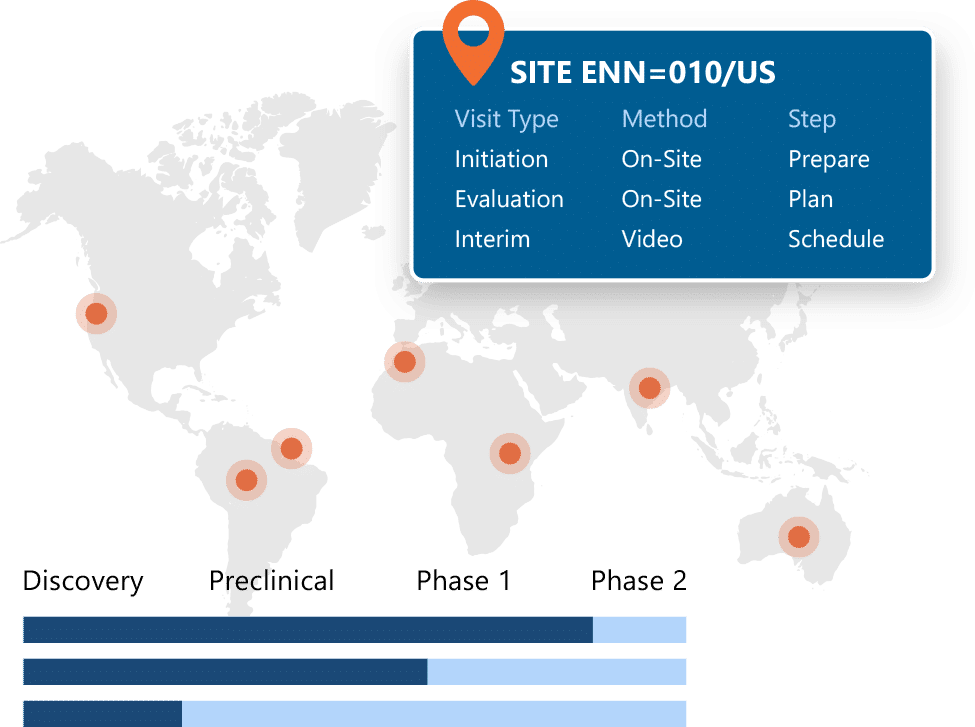
Centralized and Globally Accessible CTMS Software
Ennov CTMS is built upon the Ennov Compliance Platform (Ennov Process, Ennov Doc and Ennov Report) and leverages that power and flexibility to provide a centralized, 100% web-based solution that can be accessed by clinical teams from any place at any time. Configurable security rules control user access while proving robust collaborative capabilities to internal team members and external partners.
Ennov CTMS is fully integrated with Ennov Clinical Data Management applications. Study information from Ennov EDC can be directly imported into Ennov CTMS to accelerate study startup and eliminate the need for redundant data entry. This approach of consolidating clinical trial data into a single repository gives the trial manager a competitive decision making advantage while improving data quality and consistency.
Advanced Analytics for Risk-Based Management of Clinical Trials
True risk-based approaches require real-time data to anticipate, detect and mitigate risk. Ennov CTMS provides dashboards that unify information to clearly present status, progress, risks, and outliers.
Dashboards cover recruitment/enrollment, site and subject status, protocol deviations and CAPAs, health authority status, adverse events, queries, and much more. Many dashboards incorporate recommendations from industry sources such as TransCelerate. Users can refine the analysis to focus on specific studies, countries, investigational sites, and much more, and export with one click to power spreadsheets, presentations and reports.
Core Capabilities
- Comprehensive organization and personnel directories
- Patient recruitment tracking
- Query and deviation tracking
- Adverse event tracking
- Drug supply management
- Financial and budget trackling
- Robust monitoring capabilities
Key Features
- Centralized database
- Integrated workflow
- Comprehensive and configurable data model
- Intuitive user interface
- Intergrated with Ennov CDMS
- 100% web-based
- 21 CFR Part 11 compliant
Almedis Case Study
“For Almedis, Ennov Clinical has been the perfect match. We’ve increased general profit and market share. What’s not to like?”
Anna Torubarova,
Head of Medical Affairs Almedis
Efficiently & Securely Capture & Manage Clinical Trial Information
The Ennov Clinical suite consists of Clinical Data Management applications as well as Clinical Trial Management applications that are available for deployment in the cloud or on premises.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
