Ennov Webinar
Ennov Dashboards in Action: Visualize Your Clinical Trial Risk and Status
Tuesday, 11 May 2021 11:00AM EDT | 5:00PM CEST
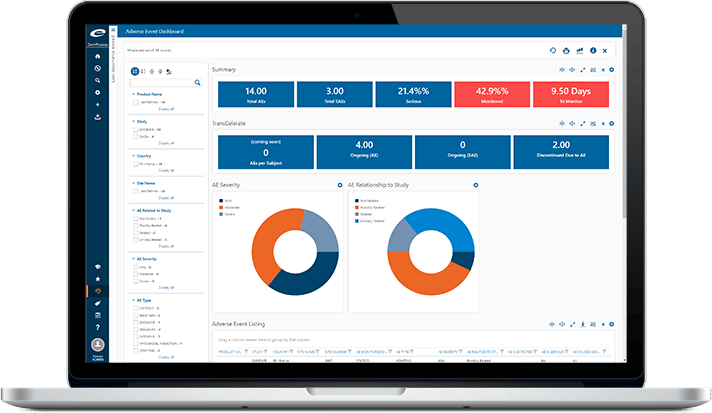
Encouraged by Health Authorities, many life sciences companies have adopted risk-based approaches throughout the organization. But effective risk management programs require data to identify the trends and outliers that can help companies anticipate and mitigate risk. Often, that data exists in multiple places, creating a burden on those who are responsible for gathering, aggregating and interpreting it.
Ennov Analytics frees you from data gathering and spreadsheet manipulation by providing targeted dashboards where you can complete a precise analysis with just a few clicks. Our preconfigured dashboards are based on trusted industry sources and can be easily modified to meet your needs quickly, by us or by you.
With Ennov Analytics, you can:
With Ennov Analytics, you can:
- Visualize your risk, compliance and efficiency
- Instantly track progress towards meeting your KPIs
- Retrieve precisely the information you need to know, with no configuration or customization
- Instantly assemble reports and figures that used to take hours to create
Our webinar will include live demonstrations and discussions to help you envision a future where key Clinical Trial information is always at your fingertips.
Once registered, you will receive a confirmation email containing information on how to access the webinar.
Ennov: An intuitive and unified content and information management platform to support and enrich the entire Life Sciences product life cycle
Ennov provides the most intuitive, comprehensive, and cost-effective suite of software solutions for the life sciences industry. From leading pharmaceutical companies to emerging biotechnology companies, we proudly serve over 200 companies and 150,000 users around the world. Our solutions are designed and built to support the entire Life Sciences R&D continuum including Clinical, Regulatory, Quality, Pharmacovigilance and Commercial. Ennov is headquartered in Paris and has offices in the US and UK.
