Ennov Document Transformation
Accelerate regulatory document preparation with DocShifter's fully automated PDF conversion and enrichment.
- Automated document conversion
- Fast, compliant, and scalable
- Submission-ready PDFs
- On-premise or cloud deployment
- Trusted by top life sciences orgs.
- 21 CFR Part 11 compliant
The Regulatory Document Preparation Challenge
Preparing submission-ready PDFs for regulatory filings is often slow, manual, and error-prone. Teams spend valuable time formatting documents, converting files, and checking quality, instead of focusing on higher-value work.
These inefficient workflows delay submissions, increase costs, and raise the risk of non-compliance. In a regulated environment where speed and accuracy matter, the impact can be significant.
If your organization faces these challenges, Ennov DocShifter offers a proven way to eliminate manual effort, improve quality, and accelerate time to submission.
Automate and Accelerate Submission-Ready PDFs
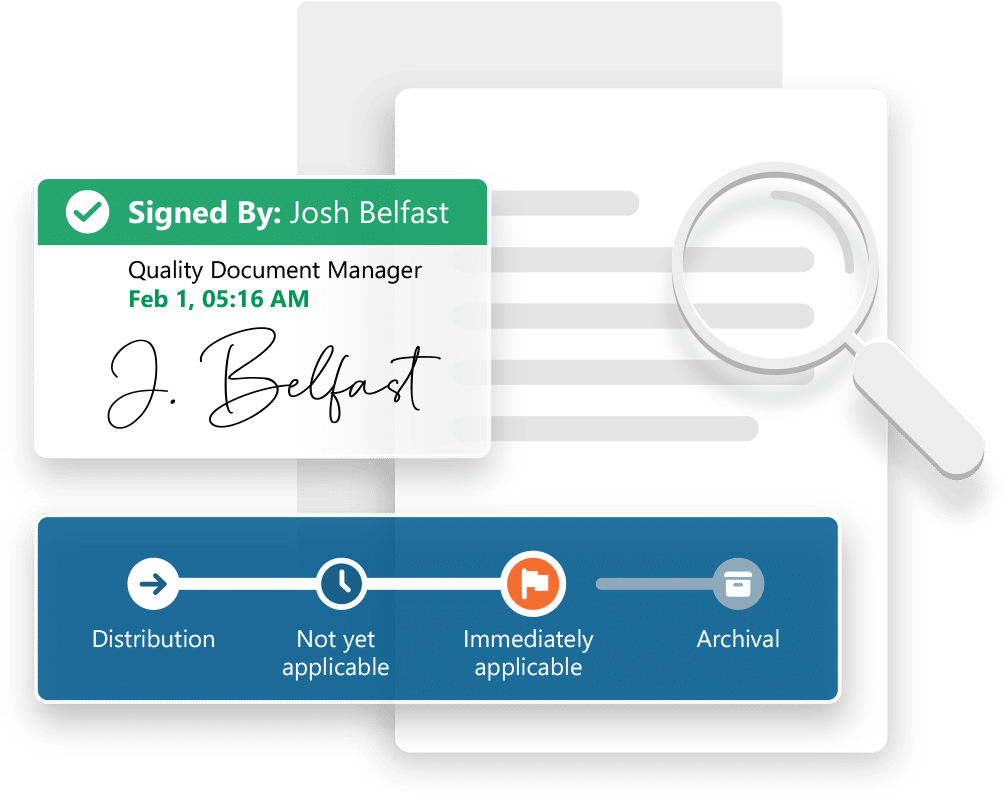
DocShifter automates the entire process of preparing compliant PDFs, including conversion, enrichment, and quality control. The result is faster, more reliable workflows that reduce manual effort and minimize risk.
It produces submission-ready documents that meet global regulatory standards, including those of the FDA, EMA, and PMDA. The platform handles high volumes with ease and integrates with your existing tools and repositories without disruption.
Whether deployed on-premise or in your cloud, DocShifter is built for speed, scalability, and compliance.
Trusted by Leading Life Sciences Organizations
For over 20 years, DocShifter has been trusted by pharmaceutical, biotechnology, animal health, and medical device companies around the world.
Built in close collaboration with regulatory teams, the platform continues to evolve to meet industry needs and stay aligned with changing regulatory requirements.
DocShifter is designed to streamline complex document conversion workflows and help your teams focus on what matters most.
Manage Complex Document Conversion with Ease
DocShifter is designed for the unique challenges of regulated industries. It supports a wide variety of document types used across clinical, regulatory, and quality functions.
Its high level of configurability and seamless integration with Ennov Doc, Ennov Dossier, Ennov Process, and other systems ensures it fits smoothly into your existing workflows.
Whether you’re preparing documents for eCTD submissions or internal reviews, DocShifter provides the flexibility and control you need.
Core Capabilities
- Automated PDF conversion and enrichment
- Integrated quality control
- Submission-ready outputs aligned with global requirements
- Scalable for high-volume workloads
- Compatible with all document types and formats
- Deployable on-premise or in your private cloud
Key Features
- Configurable conversion workflows
- Integration with EDMS, RIM, and other systems
- Automated tagging, bookmarking, and hyperlinking
- Built-in quality checks and validation
- Integrated PDF Viewer
- 21 CFR Part 11 compliant
- 100% web-based
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
