In December, FDA issued a draft guidance, Master Protocols for Drug and Biological Product Development. This guidance is currently in the public comment period; comments received to date can be viewed on its Dockets page.
FDA’s intention is to allow sponsors to accelerate drug development using well-designed and -conducted trials using master protocols, maximizing the amount of information obtained from the research effort. Compared with standalone trials under separate protocols, a master protocol may offer certain advantages by leveraging a shared control arm and other shared protocol elements (e.g., visit schedule, measurement procedures), shared infrastructure (e.g., recruitment efforts, network of clinical sites, central facilities, central randomization system, data management systems), and shared oversight (e.g., steering committee, data review committee)
At the same time, master protocols add elements of complexity, which can increase start-up time and can lead to design challenges such as ensuring adequate blinding to treatment assignment. Additionally, master protocols involving multiple stakeholders will require a high degree of coordination. Sponsors will have to carefully weigh these considerations when deciding whether a master protocol is appropriate as part of a drug development program.
In this blog post, we’ll review what a master protocol is, and the impact it may have on trial procedures and processes, as well as on the regulatory/IND process.
Overview and Definitions
Master protocol: a protocol designed with multiple substudies, which may have different objectives and involve coordinated efforts to evaluate one or more medical products in one or more diseases or conditions within the overall study structure. FDA expects that a master protocol sponsor (person or organization) will take responsibility for and initiates the master protocol. In many instances individual drugs chosen for evaluation in the master protocol will also be evaluated under separate Investigational New Drug Applications (INDs) independent of the master protocol. A sponsor responsible for the investigation of an individual drug evaluated under the separate IND is referred to as the individual drug sponsor.
Substudy: the information and design features (e.g., objectives, design, methodology, statistical considerations) related to evaluation of a single medical product in a single disease, condition, or disease subtype in the master protocol.
Examples of trial types that could utilize a master protocol include the following:
- Umbrella Trial: A trial designed to evaluate multiple medical products concurrently for a single disease or condition.
- Platform Trial: A trial designed to evaluate multiple medical products for a disease or condition in an ongoing manner, with medical products entering or leaving the platform.
- Basket Trial: A trial designed to evaluate a medical product for multiple diseases, conditions, or disease subtypes.
Trial Procedures and Processes
FDA provides details to help sponsors think through the impact that the use of a master protocol will have on many aspects of a trial. These include:
Randomization. The guidance goes into considerable detail about randomization impact. In master protocols, the randomization ratio may change over time as products enter or exit a trial. When this happens, comparisons between drugs and controls should account for different randomization ratios using approaches like stratification by time period or inverse weighting. In settings where subjects can be treated with multiple drugs simultaneously, a factorial design may be considered. In addition, Master protocols may require drug-specific eligibility criteria to ensure subject safety and the integrity of the randomized comparison.
Blinding. Achieving complete blinding becomes more complex with multiple drugs or dosing schedules. Different blinding strategies can be employed, such as multiple-dummy designs or distinct, blinded placebo controls for each drug, depending on trial design and drug development stage.
Informed Consent. The informed consent process should encompass all potential treatment arms to which a subject could be randomized. In platform trials where drugs can enter and exit over time, the consent form should be updated to reflect the current drugs under evaluation. It’s crucial that informed consent occurs before randomization and avoids substudy-specific consent to maintain comparability between different drug groups and the shared control group. Consent after randomization may result in subjects with different characteristics across substudies, jeopardizing the comparability of treatment groups. This is particularly relevant in master protocols where subjects may consent to a substudy before being randomized to a specific drug or its control, potentially leading to noncomparable groups in the shared control arm.
Adaptive design aspects.. Master protocols often incorporate adaptive design elements, such as interim analyses to potentially halt enrollment in a drug substudy due to efficacy or futility, adjust sample sizes, or modify randomization ratios. While principles from the guidance for adaptive designs in clinical trials are generally applicable, adapting them to master protocols poses unique challenges. For instance, in umbrella or platform trials, interim analyses based on blinded pooled data may lead to dissemination of comparative efficacy information if conducted separately for each drug substudy. Conversely, pooling data across all drug arms and the control arm may better protect confidentiality but may provide less accurate estimates of sample sizes needed for each drug evaluation.
Shared Oversight Committees
FDA recommends a central institutional review board (IRB) to review the master protocol, informed consent, and other relevant documents associated with trial monitoring. FDA also recommends that the sponsor appoint an independent, external data monitoring committee (DMC) or other appropriate independent entity to oversee accumulating safety and efficacy data. Depending on the trial design, the sponsor may decide to have an endpoint assessment or adjudication committee to review data on important efficacy and/or safety endpoints in the trial.
Use of shared committees complicates controlling the blind and the dissemination of data which requires well-defined governance. For example, If a shared control group is used, knowledge of blinded pooled data for the drug still in the trial (i.e., pooled across the drug and shared control groups) in addition to the comparative results reported for the first drug may lead to partial unblinding of comparative results for the drug still being evaluated. Hence, it may be important to limit access to these pooled data if results are to be reported for other drugs with overlapping control groups.
Safety Considerations
The master protocol sponsor should establish a systematic approach that ensures the rapid communication of serious safety issues to clinical investigators and FDA under IND safety reporting regulations. This should include a process for rapid implementation of protocol amendments to address serious safety issues. With regard to safety reporting, sponsors should be aware of the following:
- All clinical investigators are required to submit safety reports to the master protocol sponsor.
- Master protocol sponsors are required to submit IND safety reports to FDA and all participating investigators when they determine that a serious adverse event is unexpected, and there is a reasonable possibility that the drug caused the serious adverse event.
Regulatory Considerations
The regulatory considerations for a master protocol have increased complexity compared to those for a protocol for a stand-alone trial given the involvement of additional stakeholders, the potential for frequent changes, and the quantity of documentation. Because of these complexities, each master protocol should be submitted as a new IND to FDA.
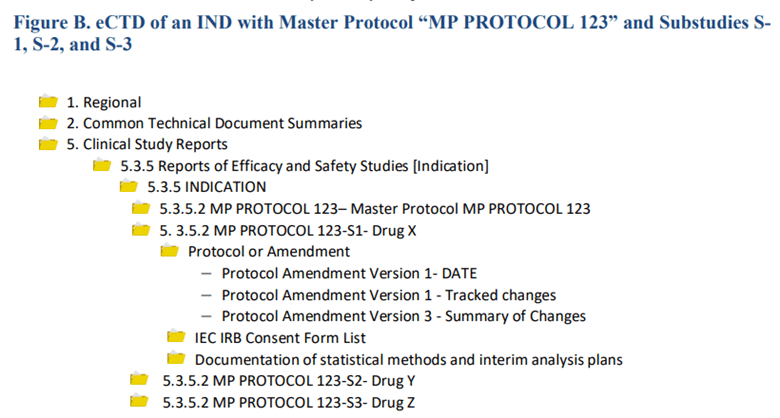
A new drug proposed for evaluation (i.e., a new substudy) in the master protocol should be submitted as a protocol amendment to the master protocol IND. For master protocols submitted electronically, FDA requires that Study Tagging Files be used to identify the master protocol and each of its substudies, as shown in the example below.
The master protocol sponsor should submit protocol amendments that substantively affect the safety, quality, or scope of the master protocol at least 30 days before initiation
A master protocol sponsor should request a pre-IND meeting to discuss the protocol and submission details.
The master protocol sponsor should provide a separate Investigator’s Brochure (IB) for each drug being evaluated in the master protocol rather than a single IB that covers all the drugs being evaluated.
FDA also provides details on IND Cross-Referencing:
- The master protocol, in its entirety, should not be incorporated into other INDs via cross reference.
- Individual drug INDs for drugs being evaluated in a master protocol can cross-reference limited elements of the master protocol IND (e.g., the drug-specific substudy).
- The master protocol IND should cross-reference information in the INDs for the individual investigational drugs, such as nonclinical study findings, drug product quality specifications, and clinical data.
TMF Considerations
The Trial Master Files of the substudies will be impacted in a number of ways. Many of these will be related to the processes and documents shared across studies, for example:
- The Master Protocol itself
- The Master Informed Consent
- Shared oversight committee documents
The TMF will have to account for the fact that these documents might continue to evolve and to have new versions after the end of a substudy, so it must be clear which versions actually apply to the substudy.
In addition, since the FDA has stated that sponsors of the Master Protocol and its substudies may be different, TMFs may be held across organizations, resulting in potential communication and data exchange issues.
Conclusion
The FDA’s draft guidance on Master Protocols represents a significant shift towards more efficient drug development processes, promising to streamline clinical trials through shared resources and oversight despite adding complexity and coordination challenges. Master protocols offer the potential for accelerated drug development by leveraging integrated trial elements, though they require careful consideration of their intricate design and stakeholder collaboration. As the industry navigates the implications of these protocols, the balance between innovation and operational complexity remains pivotal. This exploration underscores the transformative potential of master protocols in enhancing drug development, highlighting the need for ongoing dialogue among regulatory bodies and stakeholders to optimize their implementation and impact.


