Life sciences organizations face mounting pressure to accelerate regulatory processes while maintaining rigorous quality standards. Whether ensuring compliance, expanding globally, or managing complex product portfolios, success depends on implementing Regulatory Information Management (RIM) and Quality Management System (QMS) platforms that support both current operations and future growth.
Getting these implementations right requires more than just selecting feature-rich systems. It demands a strategic approach that considers how regulatory and quality functions work together in practice and ensures they have everything they need to succeed.
Best Practice #1: Design for Cross-Functional Workflows
The highest-performing life sciences companies approach RIM and QMS implementations with integrated thinking from day one. Regulatory and Quality teams may have distinct responsibilities, but their work intersects constantly.
A manufacturing change control initiated in QMS triggers regulatory variation submissions across multiple markets. A regulatory inspection requires immediate access to quality audit trails and deviation records. New product launches depend on both accurate registration data and documented Good Manufacturing Practice (GMP) compliance. All of these activities require regulatory and quality to work together seamlessly, without siloed systems creating inefficiencies and delays.
Key considerations for cross-functional design:
- Involve both Quality Assurance and Regulatory stakeholders in system design sessions from the outset
- Map real-world workflows that span both domains before configuring individual systems
- Evaluate how RIM and QMS systems will interact – whether through connectors requiring frequent maintenance or native connections on a common platform
- Align metadata standards and terminology across RIM and QMS functions to prevent confusion
- Plan for shared master data management that eliminates duplicate entry and reconciliation
When business processes naturally flow between teams, technology systems must support that collaboration seamlessly.
Best Practice #2: Prioritize Platform Architecture Over Point Solutions
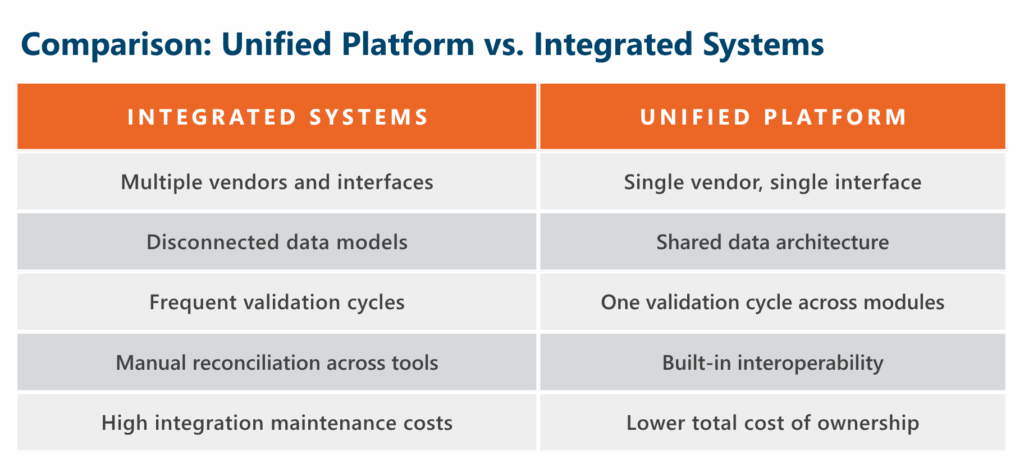
Many organizations approach RIM and QMS implementation by selecting best-of-breed point solutions for each function, then attempting to connect them through integrations. This approach introduces significant operational risk and long-term complexity.
Each integration point requires ongoing maintenance, creates potential failure modes, and complicates validation processes. When upstream systems change, downstream integrations often break. Data synchronization delays can impact critical regulatory timelines.

Key considerations for platform architecture:
- Evaluate whether vendors offer a single data model that eliminates replication and version control issues
- Look for native workflows that span domains without integration complexity
- Unified architectures can simplify validation by reducing integration touchpoints and harmonizing controls
- Assess document repository capabilities to ensure single-source version control
- Determine if the platform provides true unification rather than separate systems connected by middleware
True platform unification means regulatory and quality functions operate from the same foundational architecture, not separate systems connected by middleware.
Best Practice #3: Plan for Global Scalability
RIM and QMS implementations must accommodate organizational growth and geographic expansion. Systems that work well for single-site operations often struggle when companies expand across regions with different regulatory requirements.
Key considerations for global scalability:
- Assess whether the platform can support multiple regulatory frameworks within a single instance
- Evaluate how the system handles country-specific submission requirements and local language needs
- Determine if expanding from QMS to RIM functions (or vice versa) requires additional licensing or implementations
- Consider whether regional teams can customize workflows while maintaining global data consistency
- Look for vendors who can demonstrate clear expansion paths that leverage existing implementations
Vendors offering truly scalable platforms can demonstrate growth strategies that build upon existing investments rather than requiring parallel system deployments.
Best Practice #4: Enable Business User Empowerment
Modern RIM and QMS platforms should empower business users to adapt processes without extensive IT involvement. Regulatory and quality requirements evolve rapidly, and organizations need systems that can keep pace with changing operational needs.
Key considerations for user empowerment:
- Look for drag-and-drop workflow builders that don’t require coding expertise
- Evaluate configurable forms and data capture templates that users can modify independently
- Assess user-defined reporting and dashboard capabilities for self-service analytics
- Consider role-based access controls that business users can manage without IT intervention
- Determine if teams can own their processes and modify workflows as requirements change
When teams can adapt their systems independently, they respond faster to regulatory changes and operational requirements.
Best Practice #5: Evaluate Total Cost of Ownership
RIM and QMS implementation costs extend far beyond initial software licensing. Organizations must consider the full lifecycle expense of their technology decisions.
Key considerations for total cost evaluation:
- Factor in integration development and maintenance costs for multi-vendor environments
- Consider validation complexity – multiple systems require separate validation cycles and ongoing compliance burden
- Assess training requirements as teams work across different interfaces and systems
- Evaluate data migration complexity when systems eventually need replacement or upgrade
- Compare unified platform costs against the overhead of maintaining multiple connected systems
Unified platforms can lower total cost of ownership by reducing integration maintenance and reconciliation effort, especially when they replace multiple overlapping tools and data stores.
Vendor Selection Framework
Selecting the right vendor requires looking beyond feature checklists to evaluate fundamental platform capabilities that will determine long-term success.
Ask these critical questions during vendor evaluation:
- Platform Architecture: Does the vendor offer true unification with a single data model, or separate applications connected through integrations?
- Scalability Path: Can I expand from QMS to RIM functions (or vice versa) without additional system implementations?
- User Empowerment: Can business users build and modify workflows without vendor professional services or extensive IT involvement?
- Validation Approach: Will I need to validate one unified platform or multiple connected systems with complex integration mappings?
- Global Capabilities: How does the platform support multi-region deployments with different regulatory frameworks and local requirements?
If vendors cannot clearly demonstrate unified architecture, business user empowerment, and scalable global capabilities, you may be evaluating point solutions rather than true platforms.
Implementation Success Factors
Even the best platforms require thoughtful implementation approaches to deliver maximum value.
Critical success factors:
- Executive sponsorship that emphasizes cross-functional collaboration over departmental optimization
- Change management that prepares teams for integrated workflows and shared data ownership
- Phased rollout that proves value incrementally while building organizational confidence
- User adoption focus that prioritizes training and process optimization alongside technical configuration
- Organizations that treat RIM and QMS implementation as transformation initiatives rather than technology projects achieve significantly better outcomes.
Don’t treat security and data integrity as afterthoughts. Evaluate audit trail design, access controls/segregation of duties, encryption and backup/DR capabilities, vendor quality practices, and how configuration changes are governed and validated.
The Unified Platform Advantage
The choice between fragmented point solutions and unified platforms shapes organizational capabilities for years. Companies using integrated RIM and QMS platforms report faster regulatory responses, improved cross-functional collaboration, and reduced operational complexity.
Unified platforms enable:
- Faster decision-making through integrated data visibility
- Reduced compliance risk via unified audit trails and consistent processes
- Improved agility as teams can modify workflows without integration dependencies
- Lower operational costs through elimination of manual data reconciliation and system maintenance overhead
- As regulatory complexity increases and competitive pressures intensify, these advantages become increasingly critical for organizational success.
Moving Forward
Implementing RIM and QMS systems represents a strategic opportunity to transform how regulatory and quality functions operate and collaborate. The platforms you choose and the implementation approach you take will determine whether these systems become operational enablers or administrative burdens.
Focus on vendors that offer true platform unification, business user empowerment, and global scalability. Plan implementations that prioritize cross-functional workflows and user adoption. Measure success not just by system functionality, but by improved collaboration and faster regulatory outcomes.
The organizations that get RIM and QMS implementation right will be those that recognize these systems as the operational backbone for integrated regulatory and quality excellence.
Preparing to strengthen your quality operations? Quality requirements continue to evolve, making consistent, well-governed quality processes just as critical as initial implementation. Ennov’s Quality Management Software (QMS) provides a unified QMS to manage quality documents, training, CAPAs, audits, and all other quality documents and processes in a validated environment. By standardizing quality processes and improving visibility across teams, Ennov helps organizations enhance quality over time and remain audit-ready as regulatory expectations change.


