Navigating the complex world of electronic Common Technical Document (eCTD) submissions to the FDA can be a daunting task. Ensuring compliance with FDA regulations and avoiding potential pitfalls is crucial for a successful submission. In this article, we will explore the most common reasons for eCTD rejection by the FDA, based on insights from the FDA’s own presentations, and provide practical recommendations on how to prevent these issues from occurring. By understanding these common errors and implementing proper validation processes, sponsors can significantly reduce the risk of eCTD rejection and maintain compliance with FDA requirements.
Specifications for eCTD Validation Criteria
The FDA has been performing technical validations of eCTDs submitted to them for at least 15 years, so by now, sponsors are well aware that their submissions are subject to rejection. As a reminder, Specifications for eCTD Validation Criteria defines technical rejection criteria and specifies the consequences of errors1:
Severity Levels
The severity levels outline the impact on the official receipt of your submissions.
| Severity | Description |
| High | The error is a serious technical error which prevents the processing of the submission and will require resubmission. The submission is considered not received by FDA. |
| Medium | The error may impact the reviewability of the submission but cannot be determined without further inspection by the review staff. The submission might be considered received by FDA. |
| Low | The error is a technical error which may or may not impact the reviewability or the integrity of the submission. The submission will likely be considered received by FDA. |
Note: The FDA utilizes a commercial off-the-shelf product to validate eCTD submissions. The vendor provides the error numbers, groups, and sections. These may be subject to change from time to time. Should they change, this document will be updated accordingly. Error codes 2-7 are not validated by a commercial off-the-shelf product.
It’s surprising that the FDA still rejects a significant number of submissions – less than 2% according to a recent presentation at DIA RSIDM2, but when you consider the volume of submissions they receive, it’s still a large number. At RSIDM in 2023, the FDA’s Ethan Chen, Director, Division of Data Management Services and Solutions, FDA/CDER Office of Business Informatics, discussed the most common reasons for rejection, as shown below.
Common eCTD Rejection Reasons
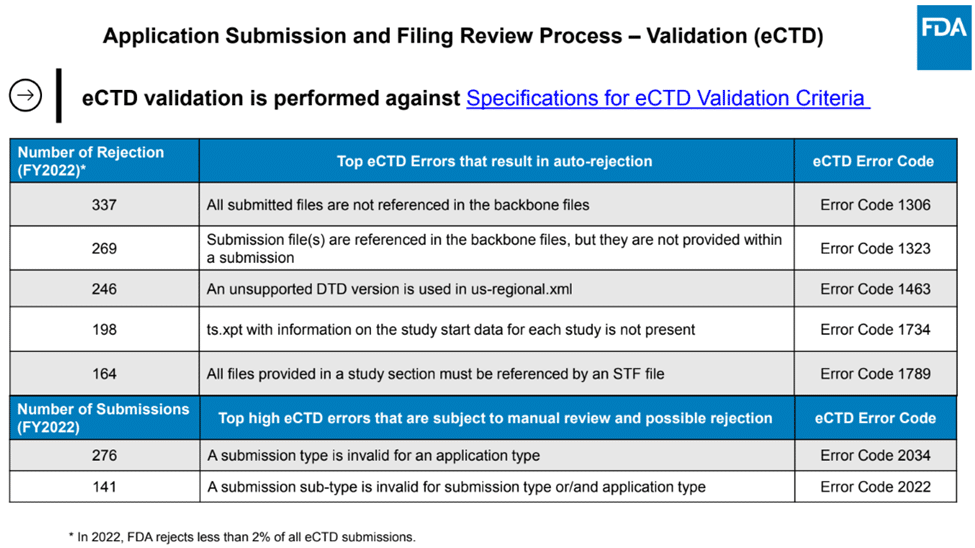
Why Do These Errors Occur?
The first five problems should be identified by any competent validation tool. So why do they occur? Possible reasons could include a bug in the tool used for validation or perhaps a failure to perform a final validation after changing the submission.
The final two errors should also be detected by a validator, but possibly some validators do not enforce correct relationships between applications and submissions.
Preventing eCTD Rejection
Given that these are the most common errors encountered, it would be a worthwhile exercise for sponsors and others preparing submissions to review these errors and ensure that their validators are detecting them properly, and that their processes always include a final validation immediately before submission to the gateway.
Enhancing Submission Success: Key Takeaways for eCTD Compliance
By addressing these common errors, sponsors can significantly reduce the risk of eCTD rejection by the FDA. Implementing rigorous validation processes, staying informed about the latest regulatory requirements, and investing in ongoing staff training can not only streamline submissions but also ensure a higher level of data integrity and reviewability. Additionally, fostering a strong collaboration with regulatory agencies and seeking expert guidance from key representative vendors in the industry can help sponsors navigate the complexities of eCTD submissions. Ultimately, these proactive measures will lead to a more efficient drug approval process, benefiting both the pharmaceutical industry and the patients who rely on their products.


