
The Challenge
Japan’s move to eCTD 4.0 has created more questions than answers for pharmaceutical companies. For one of the world’s premier pharmaceutical companies, the goal is to be ready early; well before the format becomes mandatory in April 2026. As a pioneer in the industry, they are experienced with piloting new technologies and being early adopters. But the path forward for eCTD 4.0 was full of challenges.
In the first half of 2024, only 8% of submissions in Japan were made in eCTD 4.0 format, reflecting industry uncertainty and lack of experience with the new technology. Important details were still unclear, including how to handle study reports, CVs, and review elements. Internal teams weren’t sure how to prepare their systems, and lacked experience with the brand-new submission format. There was also some confusion around the actual deadline, adding to the pressure.
Even more frustrating, the customer wasn’t always included in meetings between the PMDA and Japanese vendors. This made it harder to get answers and stay informed.
They needed a partner who could move fast, fill in the gaps, and support them through real testing. Not just theory.

Ennov is the right partner for the challenge
Ennov’s regulatory experts worked side by side with the customer’s publishing experts during a pilot project. Together, we tested real submission scenarios using the new eCTD 4.0 format for Japan. The aims? To deepen the team’s understanding of eCTD 4.0, identify gaps in the PMDA’s requirements, and refine Ennov’s eCTD 4.0 publishing capabilities.
Here’s what we did to make the process easier:
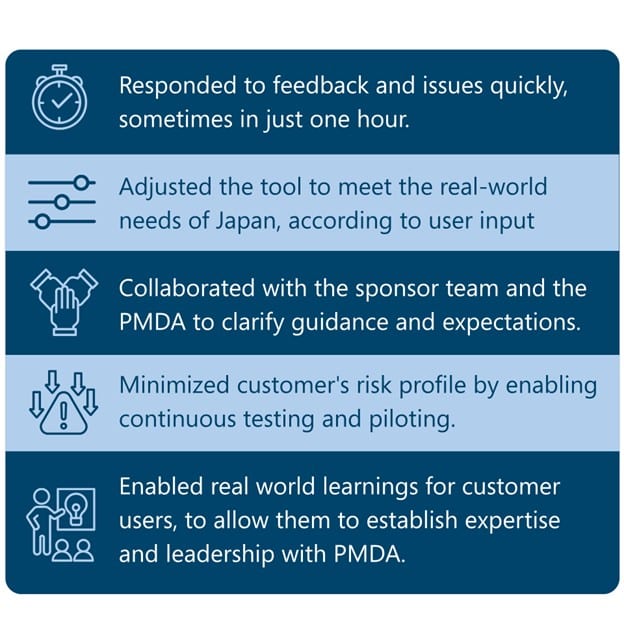
Result? Submission-Ready for 2026 (and Beyond)
At the end of the pilot, the customer was ready to put together their first full test sequence for submission to the PMDA. Because innovation is a priority and a widely accepted company policy, they chose to lead, test early, and shape the solution with their real-world experience their real-world experience in partnership with Ennov.
- They gained hands-on experience with eCTD 4.0.
- They helped improve the tool for other countries and users, too.
- They are now planning their first real submission in Japan in early 2026.
Importantly, the publishing team feels confident, optimistic, and prepared for the mandatory date in April 2026.

This customer continues to work with Ennov to improve the solution and share lessons with others in the industry. Their feedback has already resulted in new features, available to all customers, just a few months after the pilot. Their early progress proves that with the right partner, regulatory innovation is possible, even when the path isn’t fully defined.
Want to get ahead of eCTD 4.0 requirements in Japan or anywhere in the world?
Talk to our experts or explore our latest updates on eCTD 4.0 support.


