More than four years after the initial publication of the EMA’s IDMP Implementation Guide in February 2020, the EMA have taken their biggest step towards IDMP implementation yet with the go live of the Product Management Service (PMS). February 2025 will see another milestone as the EMA begin using PMS data as part of the European Shortage Monitoring Platform (ESMP) to gather information about medicine supply and demand and to prevent, detect and manage shortages of human medicines.
Marketing Authorization Holders, Sponsors, and vendors like Ennov have been preparing for IDMP for years, even as delays and reductions in scope have made implementation a moving target. The ESMP has the potential to demonstrate the value of IDMP to patients, but the implementation presents challenges for the EMA and MAHs alike.
The Challenges of Supply Chain Management
The challenges of supply chain management in an increasingly complex and interconnected marketplace were laid bare by COVID-19. Critical medicines shortages arising directly and indirectly from the pandemic rippled globally, caused by increased demand, manufacturer closures and reduced production and global delays in shipping.
The EMA stepped up its involvement in the management of shortages in the EU, establishing regulatory flexibilities to enable pharmaceutical companies to prevent and mitigate shortages, developing a common framework with member states to improve forecasting of demand data and establishing the EU Executive Steering Group on shortages of medicines.
As the critical phase of the pandemic ended, supply chain issues continued. Worldwide shortages of amoxicillin occurred throughout the winter of 2022/23, and shortages of other medications, ranging from GLP-1 agonists to chemotherapeutic agents, continue. Shortages are rarely local events: shortages in one region commonly result in a ripple effect of shortages in other regions and even in related medications as prescribers seek alternatives.
Understanding, coordinating and managing shortages is only made more complex when each health authority and marketing authorization holder (MAH) is using a different system, without any mechanism for connecting the data held by each.
The European Shortage Monitoring Platform
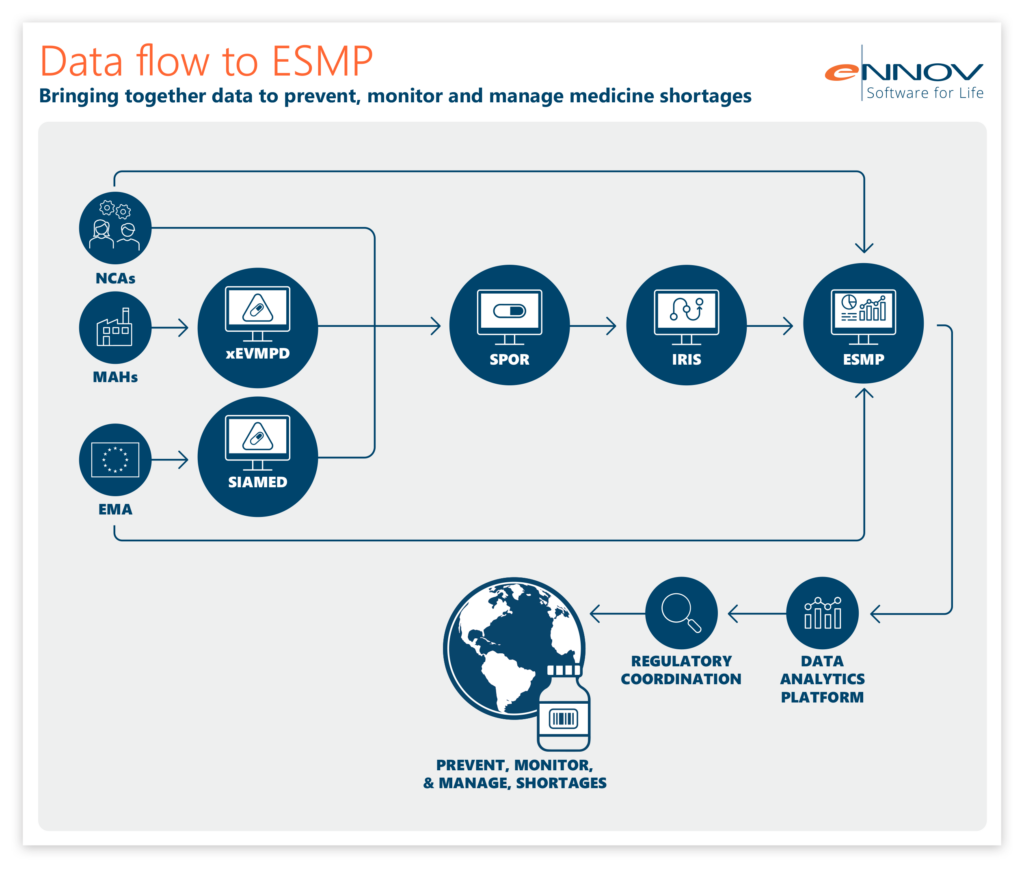
In this complex web of data, IDMP delivers unifying definitions for central concepts such as medicinal product and organization, allowing data from these myriad systems to be mapped onto one another. ESMP leverages this mapping to operate as a single platform, connecting data from national systems, the EMA and MAHs.
IDMP data definitions have been used to transform the data held by EMA in SIAMED (their internal system) and XEVMPD, mapping the two onto the data structures laid out for IDMP. Product information is made available in PMS, further enriched via an integration with IRIS (Integrated Regulatory Information System) and fed into ESMP. Data from SIAMED, XEVMPD, IRIS and national systems has been combined, using XEVMPD and PMS identifiers, allowing the EMA to understand the supply, demand and shortage risk of critical medicines in the EU. These medicines have been listed on the Union List of Critical Medicines (ULCM).
What Does This Mean for MAHs?
MAHs with medicines on the ULCM are faced with rapidly approaching deadlines for submission, review and enrichment of PMS data.
PMS is now live with data on products authorized in the EU via either the Centralized Procedure (CAP products) or non-Centralized Procedures (non-CAP products). The EMA has sourced this data from XEVMPD and SIAMED, transforming it to meet IDMP data standards and enriching it with marketing status information from IRIS before it flows into the ESMP. This process depends on submissions to XEVMPD being made at the package level, even for products where a license covers multiple packages.
Marketing Authorization Holders have therefore been asked to submit an XEVPRM for each package, following instructions provided by the EMA in Chapter 3.11: XEVPRM User Guidance. For example, licenses for CAP products are issued for a specific package and will therefore already have been submitted to XEVMPD with one XEVPRM per package. However, nationally-authorized products in Germany are authorized on a per-product basis and many MAHs will have submitted a single XEVPRM for the license and will now have to submit additional XEVPRMs so that there will be one record per package. This will ensure that data in PMS will be available at the pack set level, as required by IDMP data standards.
MAHs should also review their product data in PMS to ensure it is correct – for example, confirming that the products have been grouped, duplicated and split correctly during the migration process. The rules for this migration are extensive and are described in Chapter 7 and Chapter 9 of the EMA’s EU IDMP Implementation Guide.
Currently, data is available in read-only form via the PMS user interface or API. However, the EMA have stated that they hope to make the edit function available for non-CAP products at the end of January via the user interface, and to make edits possible via the API later in the first quarter of 2025.
MAHs should begin preparations for data enrichment, focusing initially on non-CAP products and the submission of information on pack sizes, data carrier identifiers and manufacturers. If MAHs do not already capture this information in a structured format, such as in the company RIM system, they should begin working on this urgently in order to meet the December 2025 deadline for data submission in PMS.
ESMP: A Learning Opportunity for IDMP across the Whole Portfolio
The ESMP provides a series of upcoming deadlines to spur action for MAHs who have medicines on the ULCM, but it will have valuable lessons for the rest of the portfolio. The deadlines for products on the ULCM are pressing and are followed by similar deadlines for all medicines authorized in the EU. This could represent an enormous task for MAHs with large portfolios. While it may be possible to manage the review and submission of data for a single product on the ULCM on an ad-hoc basis, this will quickly prove unmanageable for a larger list of products.
All MAHs would be wise to begin building robust processes around review of PMS data, capture of PMS data points and submission of information to PMS. Particular attention should be paid to the tools required, assessing whether this data is currently captured, where it is held, how it is maintained, and how data managers can identify and make updates.
The ESMP represents both a significant data management challenge and an opportunity for MAHs to contribute to better patient care. By leveraging IDMP standards to unify data, stakeholders can work towards more effective prevention, monitoring, and management of medicine shortages.
Preparing for ongoing IDMP change?
IDMP requirements continue to evolve, so maintaining consistent, well-governed product data matters as much as initial implementation. Ennov’s IDMP software supports scalable IDMP data management, helping regulatory teams standardise medicinal product data, improve quality over time, and stay aligned with compliance expectations as they change. Learn how Ennov’s IDMP solution helps teams stay IDMP-ready over time.
IDMP resources and implementation guidance
If you’re looking for practical materials to support your IDMP work, explore our IDMP resources hub, which brings together webinars, implementation timelines, best practices, starter packs, podcasts, and related blog posts to help regulatory teams stay aligned as requirements evolve.


