Over the last five years or so, Health Authorities have laid the framework for the collection of structured data, rather than just documents, to identify and describe medicinal products. The most significant of these standards is the Identification of Medicinal Products (IDMP) suite of standards developed by ISO. These standards are being implemented by EMA and monitored by FDA.
This internationally-agreed terminology and format facilitates the interoperability of data, improves pharmacovigilance activities, avoids duplication of encoding activities and allows easier information exchange with and between regulatory authorities. So why not build your Regulatory Information Management system around IDMP?
Want to learn more? Download our new white paper, IDMP Considerations in 2022: Interoperable Structured Data Reporting.
Pharmaceutical companies don’t live in a world structured into four convenient ISO domains, the model used in IDMP. Although the ISO organization provides a useful reference, this data structure is designed to facilitate the collection and processing of structured data by a regulatory authority. As a result, it does not facilitate business processes for life sciences companies. It is not structured for ease of data entry and retrieval, or for review and approval of data.
For example, IDMP currently only has a partial definition as a Pharmaceutical Product Identifier, not yet fully described in the EU Implementation Guidance (IG). However, re-using and reporting on a Product is of paramount importance to a pharmaceutical sponsor or MAH. Therefore, designing a RIM system around the IDMP definition of product is not practical.
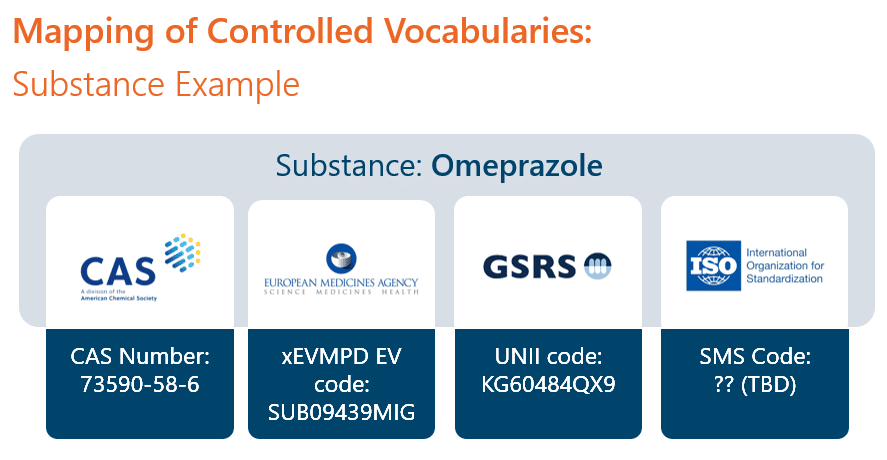
Another example pertains to the use of controlled vocabularies. Within a well-structured system, company-controlled terms should be mapped to structured data reporting terms and codes. For example, a specific substance will map to a single CAS number which is then used in CMC reporting. The same substance will also map to an xEVMPD EV code which will be submitted to the EMA in the composition. If the same product extends to the United States, the substance should map to the underlying UNII code. IDMP will map the same substance to an SMS code. In a well-designed RIMS, a user would enter or search for the company’s term for the substance, and other mappings would be automatically available and used where needed.
Fortunately, data can be modelled within a pharmaceutical RIM system to bridge these gaps. RIMS must be independent but compliant with the regulatory guidance constraints in terms of objects, class definition and data relationships (cardinality). This is a technical way of saying that as long as your RIMS is well structured, then the creation of the structured data message for reporting is a simple translation of your internally accepted terms. When guidance changes, your RIM system can accommodate it by simply re-mapping or adding data elements.
Following this train of thought, regulatory guidance will evolve over time but should not be permitted to dictate core RIM data structure. After all, the guidance for structured format and reporting represents data from the Regulatory Authority’s point of view, not the sponsor’s.
Want to learn more? Download our new white paper, IDMP Considerations in 2022: Interoperable Structured Data Reporting.


