In this fireside chat, Hal Mann and Dave Petrich, MBA, RAC discuss the evolving regulatory landscape facing CLIA labs and IVD startups, sharing practical guidance on maintaining data integrity, streamlining processes, and preparing for stricter requirements. They also explore how AI-enabled digital tools are reshaping compliance and supporting scalable growth.
Key topics include:
- The role of AI in modernizing compliance and regulatory workflows
- Today’s biggest regulatory challenges for CLIA labs and IVD startups
- Common areas of confusion and operational bottlenecks
- How smaller organizations experience regulatory pressure differently
- Impacts on time to market and inspection readiness
Watch the session below:
Highlights from the Session:
FDA Enforcement Discretion of LDTs Timeline
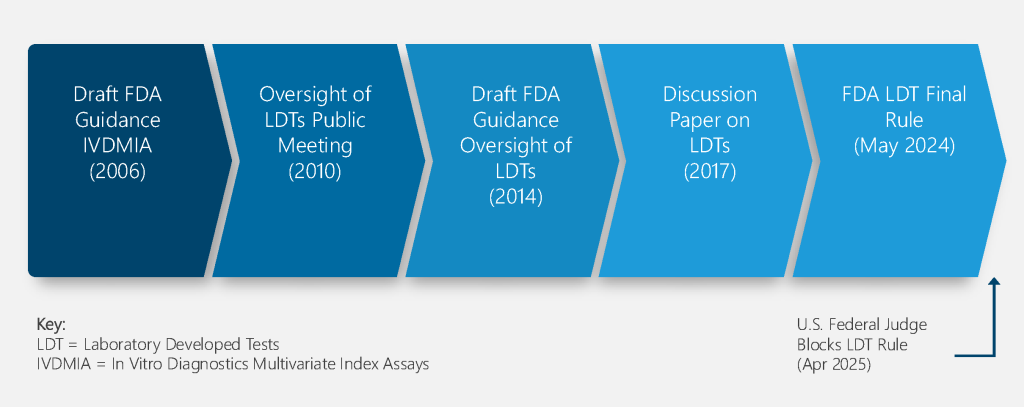
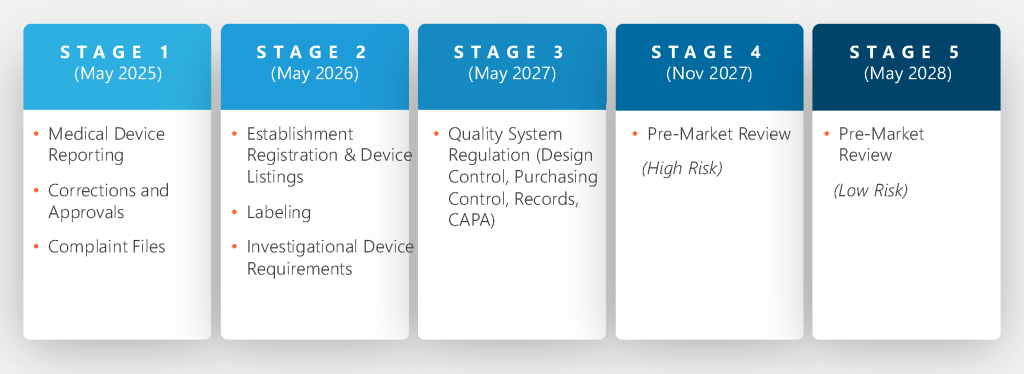
FDA LDT Rule Transition Period
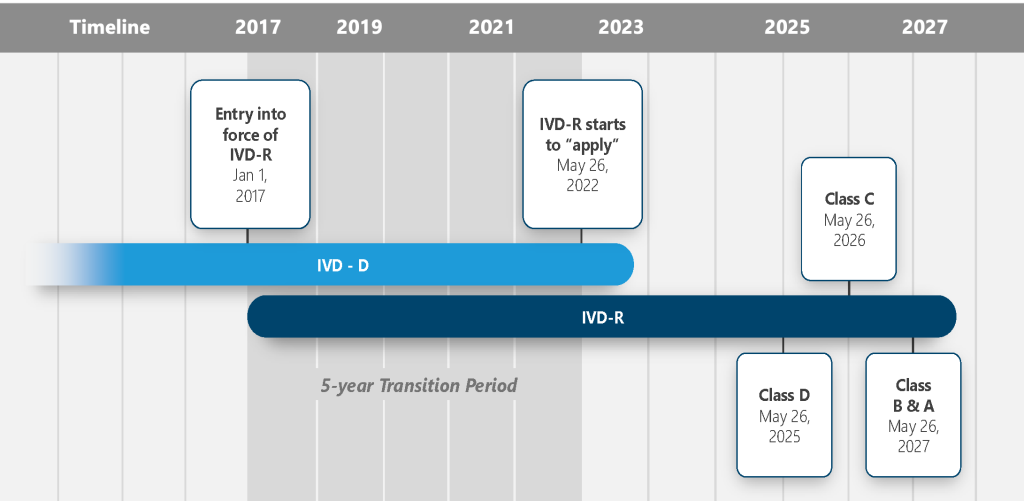
EU IVDR Transition Timeline
Revised January 2022
Key Takeaways:
- Develop strategies early in test or product development cycles to navigate regulatory requirements, including QMS implementation, analytical/clinical study plans, regulatory submissions, and inspections.
- Data integrity and traceability are paramount for demonstrating safety and efficacy (performance) for Lab Developed Test (CLIA Labs) and IVD kits (Traditional IVD Manufacturers.
- Proactively planning studies and leveraging in-house data (and external RWE) could significantly reduce the time and cost of performing analytical and clinical studies to meet regulatory requirements in multiple jurisdictions.
- Digital solutions offer advantages to improve the efficiency and effectiveness of managing data and other information, including AI-enabled solutions and electronic regulatory submissions.
Other Valuable Resources:
- The Ennovation Podcast
Get the latest trends, insights and expertise in Life Sciences and Regulatory Affairs. - LinkedIn Newsletter
News, announcements, events, webinars - Ennov Insider
Take advantage of the knowledge and best practices gained from more than 25 years of research, innovation and development for the health and life sciences sectors.


