RIM stands for Regulatory Information Management, a simple acronym for a complex task! RIM data spans many domains in life sciences but can be broadly defined in two categories: information about regulatory activities, and information about the company’s products.
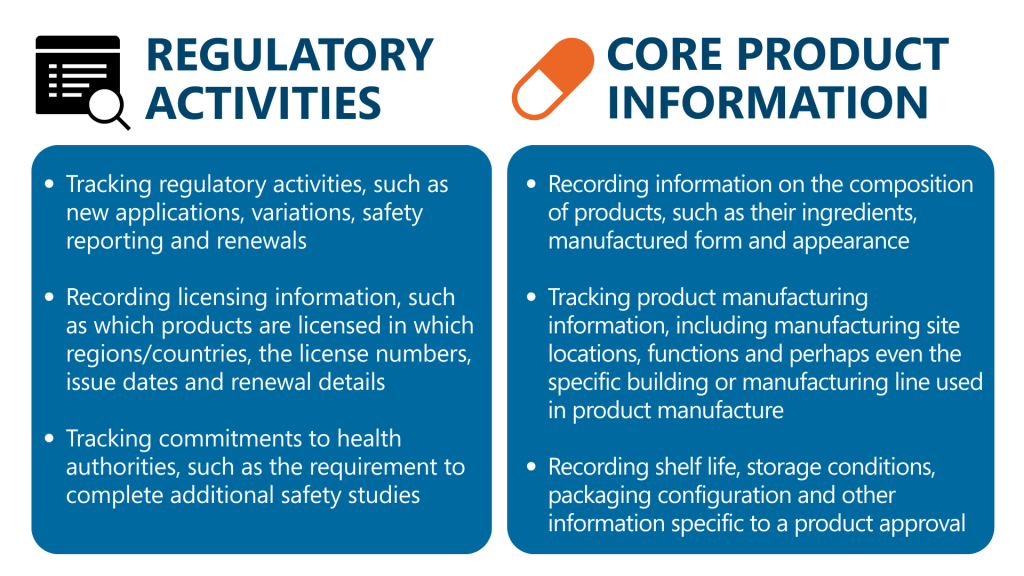
All of these areas are critical for a company to maintain compliance with health authority regulations worldwide.
What Does RIM Actually Do? Why Is RIM Important for Life Sciences Organizations?
RIM is a core part of every life sciences company’s regulatory responsibilities. Accurate Regulatory Information Management is vital to ensure companies submit renewals on time, track and meet health authority commitments, can execute changes in their manufacturing processes and much, much more.

What RIM Is Not: Common Misconceptions
It could be easy to see the data-gathering aspects of RIM as simple administrative add-ons to other tasks: important, yes, but more like filing your paperwork than really enacting change. But that would be a serious mistake. RIM isn’t a collection of spreadsheets, or even a purpose-built system: it’s a comprehensive suite of processes to gather, analyze and deploy information about a pharmaceutical company’s products and activities.
What Are the Core Functions of a RIM System?
Product Data Management
Product data management includes recording a wide range of data on a company’s product portfolio. We can divide this into three levels – foundational data, application and license information and registered product details.
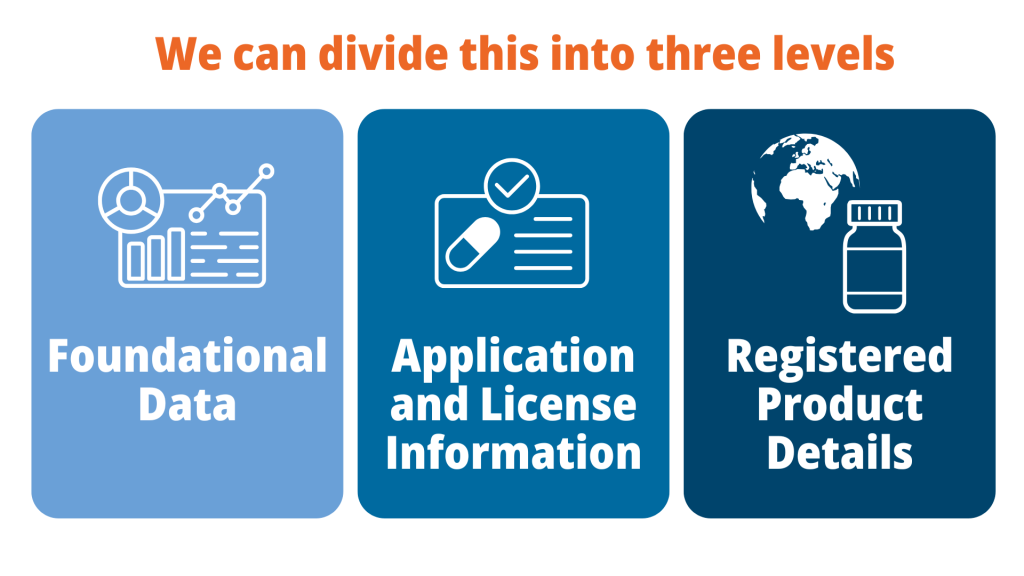
Foundational data
This forms the foundation of your RIM data. This will include information about:
- How the product is formulated: active pharmaceutical ingredients, dosage form, inactive ingredients and other formulation information.
- How the product is used: such as therapeutic indications or routes of administration.
This information may be adapted as relevant for the product in question. For example, for a cosmetic product, the active pharmaceutical ingredients may not be relevant, but the formulation will be. For a medical device, the key consideration might be the mode of action.
Application and license information
From this foundational data, the RIM system then captures where and how the product is licensed.
- Each application to a health authority will be recorded, including the details of how the product is manufactured, formulation, labelling etc. These form the registered details for the product in that specific location.
- The license, or registration, details will also be recorded, including information on if and when the license must be renewed.
- The marketing information, such as when a product was first marketed and the current marketing status, will be captured for each license, or even each registered package.
All of this information is critically important. Application information provides the company with an overview of where each product has been submitted for licensing, while license information shows where a particular product is authorized. Renewal details are used to ensure that renewals are planned, and that licenses are not allowed to expire – or worse, that products are not released to the market without a valid license.
Product lifecycle information
During the lifecycle of a product, many regulatory activities will be undertaken.
This includes:
- Scheduled submissions such as renewals and periodic safety reporting
- Extensions to the therapeutic indications
- Variations to the registered details: companies may choose to change manufacturing sites, adjust the formulation of the product, or extend the shelf life.
Efficient change management is dependent on efficient RIM systems: tracking the requirement to submit each change for approval, recording when a submission has been made, and reporting the approval promptly as it’s received. Tracking these activities provides both a vital history for each application, and ensures that each change is implemented only when it is approved by the local health authority.
Submission Planning & Tracking
Submission planning and tracking is an essential part of the submission process. Ensuring that all submissions are planned in the RIM system allows for proper forecasting of work and resource management.

RIM systems with robust reporting functionality will allow planned submissions to be easily visualized, including highlighting submissions that are delayed or overdue.
Submission tracking isn’t just for planning – historical submission information is important too. Without a record of past submissions, teams are left wading through dossiers, trying to decipher the exact topic, establish the submission date and then understand the relationship that submission has to any changes in product information. That might be feasible for one application, but it would quickly spiral into an endless task when trying to understand similar submissions made across multiple applications.
Instead, use the RIM system to track submissions, link together submissions made as part of a project, and report on those submissions. A well-managed RIM system will make answering questions such as ‘when did we submit the first wave of marketing authorization applications for our new product’ a matter of minutes, rather than days.
Health Authority Correspondence
Managing health authority correspondence is closely linked to submission planning and tracking. Correspondence might contain:
- Requests for information
- New commitments
- Other critical information that will prompt the company to take action.
While the correspondence itself will be stored in the document management system (DMS), the resulting action should be recorded in the RIM system. RIM systems should have the ability to record that a piece of correspondence was received from the agency, link to that correspondence in the DMS, and then manage the actions that arose as a result. For example, if the agency issues a request for information in response to a submission, the RIM system should allow that request to be tracked, link to the letter in the DMS, and then allow the response submission to be tracked, usually directly related to the activity tracking for the parent submission.
Commitment tracking is a critical compliance activity for life sciences companies. Commitment management is frequently audited, and failure to meet commitments would expose a company to significant risk. Health authorities may issue commitments as a condition of approval, during periodic safety assessment reporting or on an ad-hoc basis. These may range from short-term, simple requirements to long-term, complex and structured commitments.

RIM systems are vital for managing health authority commitments. Using a RIM system to track commitments minimises compliance risk by:
- Ensuring all commitments are centrally held and can be easily tracked and reported on
- Using workflow functionality to generate reminders as deadlines approach
IDMP/xEVMPD Data Preparation and Submission
The wealth of product information tracked in a RIM system is key for maintaining compliance with xEVMPD and IDMP requirements.
xEVMPD
Product information must be submitted to xEVMPD, the Article 57 database, for all medicinal products authorized in the EU and EEA – it’s a legal requirement, and comes with specific timelines for different activities. The RIM system will be the source of this key information. When it comes to submission, there are a few options:

- Data from the RIM system may be manually entered through the EMA’s EVWeb portal
- An XML message for submission to xEVMPD (called an xEVPRM) may be prepared by the RIM system but not submitted using the RIM system
- The xEVPRM may be prepared by the RIM system and submitted directly to xEVMPD.
Monitoring xEVMPD compliance is a must. Whether the RIM system is used to submit directly to xEVMPD or not, tracking your submissions in your RIM supports compliance by making this data available for reports.
IDMP
IDMP increasingly requires product information to be submitted to global health authorities. The EMA have been leaders in this respect, requiring the enrichment of data in their Product Management Services (PMS) database. This information should be stored and managed in the RIM system. An IDMP-compliant RIM system is essential to ensure that the enormous number of IDMP data points can be captured. Equally critical are the processes surrounding this data, ensuring that it can be kept up to date and reviewed for accuracy.
Submission of IDMP data remains an evolving topic. Currently, EMA allows enrichment data to be submitted directly through the PMS user interface. They hope to launch their write API service, which will allow machine-to-machine connections, in Q4 2025. This will allow RIM systems to submit data directly to PMS, which will be far more efficient than entering data manually. It will also reduce the risk of manual data entry errors, and should offer significant advantages, particularly for life sciences companies with large European portfolios. RIM systems that are ready for this functionality, with clear plans to incorporate the write API as soon as it is available, will allow life sciences companies to quickly take advantage of these opportunities.
Who Uses RIM (And Why It Means Different Things to Different Roles)
Regulatory Information Management, and RIM systems, are used throughout life sciences companies, but it means different things to different people.
There are three primary models for how RIM is integrated into a company’s workflows:
- Centralized: one team, usually in Regulatory Operations, will enter information into the RIM system.
- Decentralized: responsibility for entering information into the RIM system is split out across the company, with each team entering data that they have available.
- Hybrid: some data is entered directly by the teams who own it and other data is provided to the RIM team to enter. Typically, they will be responsible for entering complex data, such as IDMP-compliant product information, allowing them to ensure data accuracy and freeing up other teams to focus on their work.
Regulatory Affairs
Regulatory Affairs predominantly use RIM to plan, track and report on submission activities. Whether that’s planning which renewals are due this year, tracking commitments from a health authority, or reporting when submissions have been made or an approval received, RA will need to use RIM systems to ensure that the company’s regulatory activities are recorded.
Regulatory Operations
Regulatory Operations teams usually include the team responsible for maintaining the RIM system. They may or may not do day-to-day data entry, depending on the way RIM is implemented in the company, but they will certainly be responsible for training, processes, and ultimately data quality control. Typically, they will also be involved in providing RIM data to other teams: producing reports, managing integrations with other systems and managing the flow of data throughout the company. They will also be focused on compliance: from xEVMPD to IDMP, to EUDRAMED and GUDID, the ability of the RIM system to capture and submit compliant data will be critical for these teams.
Other Regulatory Operations teams, such as publishers, will use the RIM system much like Regulatory Affairs, to record their submission activities.
IT/Technology Leaders
IT and technology leaders are key stakeholders in RIM systems – responsible for coordinating implementation, upgrades and maintenance. They will also be responsible for understanding the IT landscape in their company: how each system that the company uses interacts with others, and ensuring that integrations are maintained where necessary.
IT and technology leaders may not use the RIM system in their day-to-day work, but they will be intimately familiar with its technical requirements and security. It’s important to consider how the RIM system will fit within the broader range of systems and tools available at the company. Platform solutions, which span the entire product life cycle, reduce the number of vendors that the IT team has to work with, saving time and money. Managing one system that serves multiple teams, from Regulatory to Quality to Clinical, offers significant efficiencies for IT.
Quality/Compliance Teams
Quality and compliance teams work hand in hand with regulatory teams – the RIM system must support this collaboration. Change controls initiated by the quality team, such as a change in shelf life for a product, will result in regulatory activities that need to be tracked in the RIM system. This information will then need to be fed back to the Quality team to allow them to implement changes efficiently.
Finally, it’s vital to ensure that the RIM system meets the compliance requirements of the company and of regulatory authorities. RIM systems should offer audit trail functionality, to ensure changes are traceable. We have also discussed how reporting can support compliance throughout this article: whether reporting on deadlines for health authority commitments, clearly identifying when a quality change may be implemented, or alerting stakeholders to missed compliance deadlines.
What Problems Does RIM Solve in Pharma & Biotech?
RIM systems ensure that data is collected, managed and effectively used throughout the company. What happens if the RIM system isn’t working effectively, or if there isn’t one in place?
Siloed systems
First, regulatory data ends up in silos. Companies without effective RIM systems often find themselves using a patchwork of Excel spreadsheets, ad-hoc notes or even hand-written records to keep track of product information, submissions, and regulatory activities.

This information isn’t easily accessible to others in the company, or even within the wider regulatory team. Data is often stored in multiple places, by multiple teams, leading to confusion over which of these is the source of truth. Trying to track down any information is hugely inefficient, as information has to be reassembled from these disparate sources.
Compliance risk
This patchwork of systems also poses a significant compliance risk. Without effective centralized tracking and good reporting, the risk of missing a deadline is high. For example, if each local regulatory team tracks their own upcoming submissions, a central publishing team can’t easily forecast their work. This could lead to missed submission deadlines if the publishing team don’t have the resources they would need to complete an unexpected surge of publishing requests. Worse still, if those submissions relate to health authority commitments, the company could find themselves missing a key compliance deadline.
What does an effective RIM look like?
In contrast, a company with an effective RIM system has all their regulatory information in one place. The RIM system serves as a single source of truth, and the data is easily available to all stakeholders. Regulatory Information Management processes ensure that data is only entered into the system once, without the need to re-enter it into various spreadsheets, reducing effort and risk of data entry errors. Data flows to where it’s needed, with reporting ensuring that key stakeholders are kept up to date.
How RIM Supports Regulatory Submissions
Your RIM system is an essential part of planning, supporting and preparing regulatory submissions.
Whether your RIM includes a publishing solution or not, RIM systems should be used to plan your upcoming submissions. A good RIM system will allow you to report on your planned submissions, helping to forecast workload for your publishing team, provide workflows that assist in coordinating submissions and report on overdue submissions, ensuring compliance with health authority deadlines.
It’s also important to consider data submissions. Data submission is a critical regulatory obligation for life sciences companies, encompassing xEVMPD reporting to the EMA, UDI (unique device identifier) reporting to a number of global health authorities, and, increasingly, IDMP data submission.
xEVMPD, IDMP and UDI data submissions may be made either manually through the health authority’s portals, or, in some cases, through direct connections with the RIM system. In all cases, the source of data is the RIM system.
RIM systems can further support submissions by using workflows to trigger notifications when submissions are due, ensuring compliance.
RIM vs eDMS: What’s the Difference?
By now, you probably understand that RIM refers to data. But much of that data is held in documents – from submission dossiers to agency correspondence. So where do you keep those documents? That’s where an electronic document management system (eDMS) comes in.
While eDMSs aren’t part of RIM, they’re a critical partner. Readily available documents, such as product information leaflets, ensure that RIM data can be entered and checked promptly. Delays making those documents available can really slow down the process of updating your RIM, leading to compliance issues.

It’s also important that the RIM system can easily reference documents, so that critical documents can be directly linked to the relevant records in the RIM. If you want to submit to xEVMPD directly from your RIM, you’ll need to be able to link the labelling documents, such as the summary of product characteristics, in order to submit these to xEVMPD.
Some RIM systems allow documents to be directly uploaded. However, this poses compliance risks – what if you upload the document, and then changes are made? Instead, best practice is that the RIM system should link to the document in the eDMS, which avoids duplication of documents and allows access to the eDMS version history. Ideally, the RIM and eDMS are integrated, making linking documents and RIM data effortless.
What to Look for in a RIM Software Solution
Convinced of the need for a great RIM system? At the heart of that is the right RIM software choice.
- The right RIM will act as a single source of truth – your company won’t need to use spreadsheets, paper records or email reminders.
- Look for systems with a broad scope, to cover all common use cases, paired with flexible configuration that can be adapted for your company’s specific needs.
- Data governance is one of the central tasks in regulatory information management, and it’s important that your RIM system makes this as easy as possible.
- How easy will the system will make compliance with XEVMPD and IDMP? Avoid systems where data needs to be re-entered.
- Look for systems with workflows that ensure the right data is captured throughout each process, notify users to prompt them to enter data, and offer reports to highlight potential data issues. These will ensure that data quality is maintained, and avoid your company having to do complex data clean up projects.
- The RIM system you choose should also fit seamlessly into the existing range of IT applications at your company.
- How will the RIM system will integrate with Quality, Clinical, and Pharmacovigilance systems?
- Platform solutions with shared data repositories will provide integration by default.
- Reporting is just as important to your data flow as integration. Your RIM system should have robust, flexible and easy-to-use reporting built in.
- Will you need to integrate with other reporting solutions? Data-as-a-service (DaaS) will allow you to combine data from multiple systems to create reports. If your company has a data warehouse, or uses DaaS for other systems, be sure to check your RIM vendor can offer this too.
Common Challenges When Implementing RIM Systems
Now that you’ve selected your RIM system, you need to implement it. It’s key to consider change management and user adoption right from the start of your implementation project, and even at the vendor selection stage. After all, if nobody uses the RIM system, it won’t fulfill its promise.
Consider the problems that your current RIM system – or lack of it – is causing, and identify how your new system is going to resolve these. If there are places that your previous system suits your users more than the new system, be honest about this, and make it clear to users why you decided to implement the new system anyway.
Early engagement with key stakeholders can help you sell the change to your wider user group – these early adopters can both shape the implementation to ensure you meet their colleagues needs, but also explain to those colleagues why the system acts as it does. They can also act as invaluable informal support when you go live, taking advantage of their early experience with the system to support their colleagues.
On the technical front, don’t underestimate data migration and validation effort. These aspects of the project are sometimes overlooked, and can end up being much more significant than initially forecast. Ask your new vendor about their plans and experience in data migration, and their validation support. An experienced team with the right tools can ease this burden significantly.
What’s Next: The Future of RIM
Future-proofing your RIM is about more than just making sure the vendor will align with new regulatory requirements. Regulators are demanding that life sciences companies gather more and more data, and submit that data in structured formats such as IDMP. This offers both challenges and opportunities.
Automation and AI
As life sciences companies gather more and more structured data in their RIM systems, this offers automation opportunities. This covers simple to increasingly complex, AI-powered use cases. Let’s take the example of a license that needs to be submitted for renewal. We’ll track the license expiry date, the activity for our license renewal, and then the new license information in our RIM system.
A simple automation would be to automatically create the renewal activity at a pre-determined time from the license expiry date. For example, we might ask the automation to create the activity six months before the license expires. The automation could also notify a group of users that the new activity has been created, and that the renewal needs to be organized.
A more complex automation might use AI to assess the time it has taken for previous renewals to go from ‘submitted’ to ‘approved’ and adjust the time period that triggers the creation of the new activity accordingly, ensuring work starts in plenty of time.
Structured data submission
If structured data is included in the renewal process, such as a submission to xEVMPD following approval, the RIM system will be used to make this submission. This structured content submission should be pulled seamlessly from data already entered in your RIM, without the need to re-enter information just for xEVMPD. As IDMP comes into play, a future-proofed RIM will offer more structured content authoring.
Integrated regulatory intelligence
Integrating regulatory intelligence offers further opportunities for RIM. Imagine that the health authority has changed their requirements for renewals. An integrated regulatory intelligence solution could add a reminder to the activity that the requirements have recently changed, prompting regulatory affairs colleagues to ensure that their submission meets these new requirements and avoid questions. AI-powered chatbots could even be integrated into the RIM system, allowing colleagues to ask the chatbot about the new requirements, help them find company guidance on preparing renewals, or identify similar renewals completed in the last few years so that they can use them as templates.
Frequently Asked Questions About RIM
“Is a RIM system required by regulators?”
Regulators don’t require your life sciences company to have a RIM system, but they do require you to manage your regulatory data! It’s the outcomes, not the methods, that are required: submitting renewals on time, knowing which products you have registered in the market that they regulate, etc. A RIM system will help you meet those requirements efficiently, and it offers many other benefits that we’ve discussed.
“Can RIM replace my document management system?”
Document management is an important part of regulatory information management, but your RIM system certainly won’t replace your DMS! RIM systems are more focused on data management, and DMSs are focused on the documents that contain that data. The two should work seamlessly together – integrated platforms that operate both as a DMS and a RIM system will ensure that your data and documents are interlinked.
“What’s the difference between a RIM system and a tracking spreadsheet?”
Technically, a tracking spreadsheet is a RIM system – it just isn’t one that many people would recommend! A full-featured RIM system will offer an enormous number of benefits above using tracking spreadsheets, from data reporting and analytics to automation, process management and structured content submission.
“Do I need a separate system for IDMP?”
Ideally, your RIM system should be fully IDMP-compliant and your vendor should be developing new features to allow you to submit IDMP data as health authorities begin requiring it. However, if your RIM vendor isn’t offering that, you may need to consider using an additional system for IDMP. Look for vendors that are flexible and nimble – IDMP has evolved significantly as health authorities have implemented it, and your IDMP solution needs to be able to evolve just as fast to remain compliant. In the long term, its always better to have a RIM system with integral IDMP features, rather than two separate systems.


