Ennov Clinical Suite
A total solution to capture and manage Clinical Trial information
Ennov’s Clinical Suite provides a unified, end-to-end platform to manage every stage of the trial lifecycle:from planning and data capture to oversight, reporting, and close-out.
The suite includes:
- Ennov EDC, RTSM, and ePRO for clinical data capture and patient engagement
- Ennov CTMS and eTMF for trial oversight and document compliance
- Ennov eLearning to support study team training and readiness
All solutions are designed to work together out of the box, and can be deployed in the cloud or on premises.
Benefits
- One unified platform
Manage clinical data, documents, workflows, and oversight within a single, integrated suite. Simplify operations and eliminate silos across systems and teams. - Fewer manual tasks, greater productivity
Automate repetitive processes—like data validation, site payments, and monitoring visit reports—to free up time and reduce risk of error.
- Designed for teams of any size, anywhere
Whether you’re running one study or dozens, Ennov supports collaboration across regions, teams, and partners—with intuitive tools and multilingual support built in. - Real-time visibility, better decisions
Access dashboards and analytics that provide actionable insights into trial progress, site activity, and compliance risks—before they become costly problems.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
Almedis Case Study
“For Almedis, Ennov Clinical has been the perfect match. We’ve increased general profit and market share. What’s not to like?”
Anna Torubarova,
Head of Medical Affairs Almedis
EDC
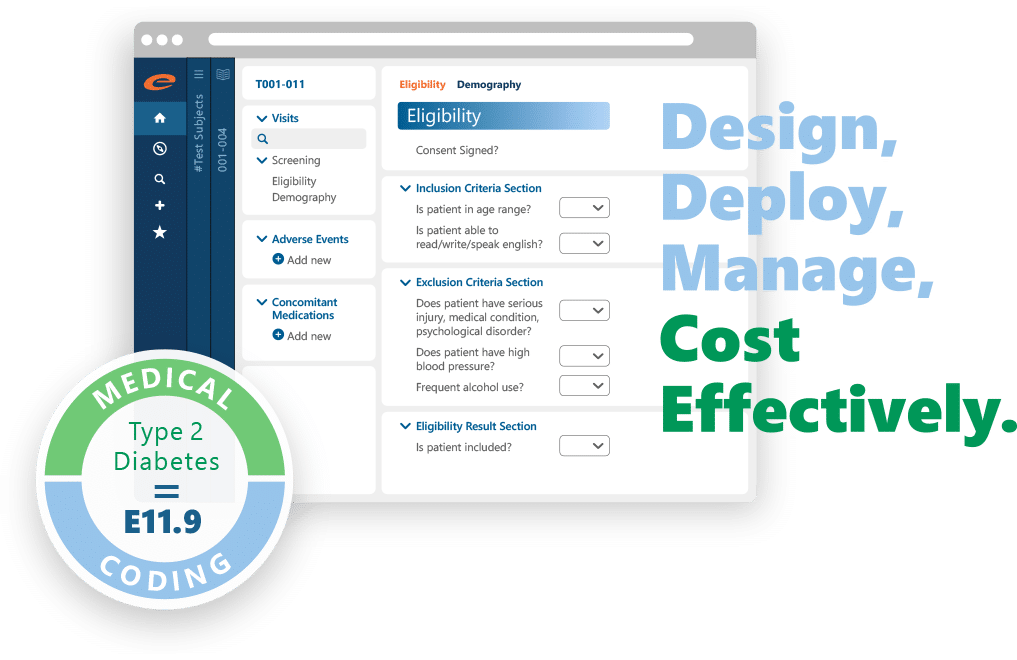
Capture cleaner data. Move faster. Spend less with a compliant eCRF software
Ennov EDC makes it easy to design, deploy, and manage eCRFs for trials of any size: from single-site studies to global Phase III programs. With built-in compliance, advanced validation, and seamless ePRO integration, you can collect high-quality data without delays or rework.
Why teams choose Ennov EDC:
- Intuitive, no-code study design and eCRF configuration
- Built-in validations and edit checks to improve data quality
- Seamless integration with Ennov ePRO for direct patient input
- Automated randomization and medical coding (MedDRA, WHO Drug)
- Fast data imports, clean exports, and reliable traceability
- Supports rapid deployment and lower study build cost
ePRO
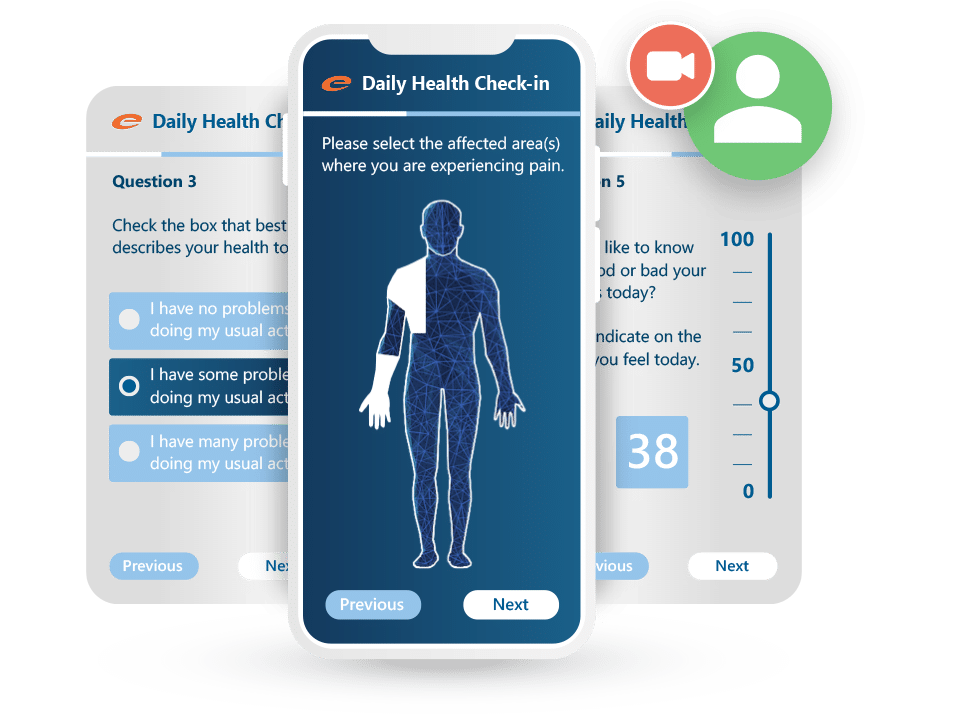
Patient-reported data. Captured cleanly, submitted securely.
Ennov ePRO makes it easy for patients to complete diaries, questionnaires, and quality-of-life assessments from any device: no paper, no delays, and no data loss. Designed to boost compliance and streamline data collection, it works hand-in-hand with Ennov EDC for a unified clinical data workflow.
Key benefits:
- Intuitive, mobile-friendly interface for patients
- Supports diaries, PROs, QoL assessments, and visit-based forms
- Real-time integration with Ennov EDC
- Automated notifications and reminders improve compliance
- Multilingual and configurable for any study design
- Fully validated and compliant with regulatory expectations
Ennov CTMS
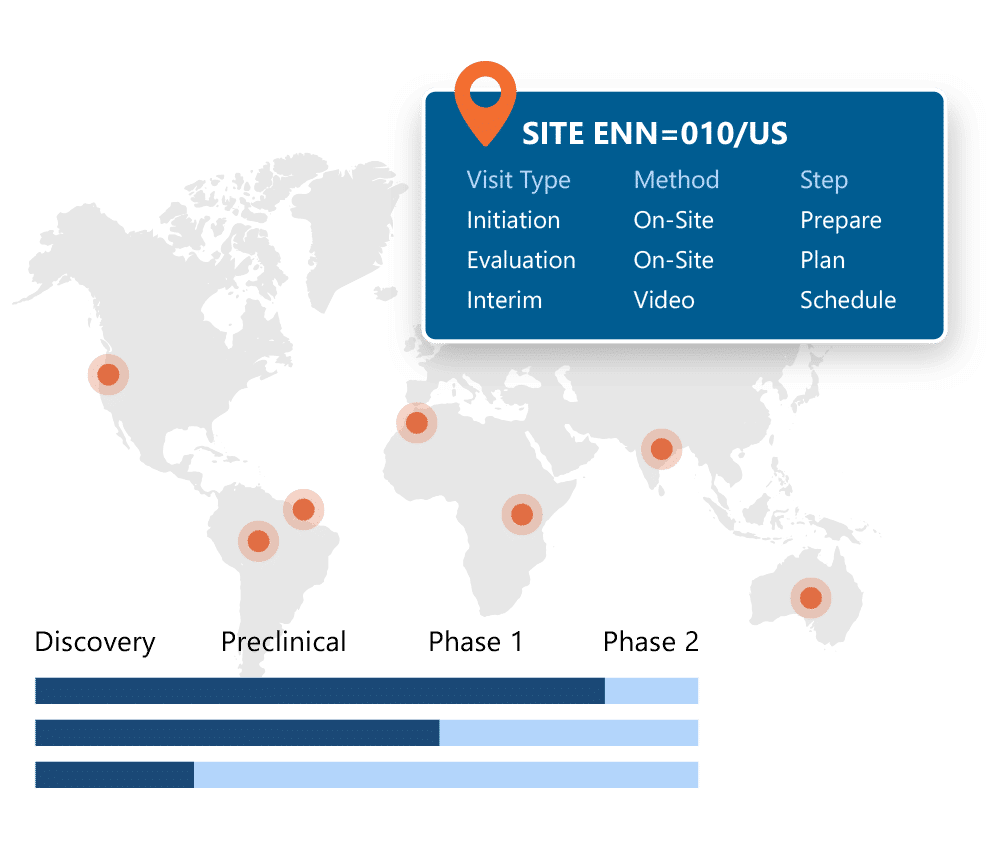
One system for trial oversight, monitoring and financial management.
Ennov CTMS gives you full visibility into your clinical trials: across countries, sites, and programs. From milestone tracking to site payments and CRA workflows, everything is built in and designed to work out of the box with Ennov EDC and the rest of the Clinical Suite.
Key benefits:
- Financial management and efficiency through accurate, on-time investigator payments based on EDC data
- Monitoring efficiency with comprehensive monitoring tool that has process and document management at it’s core.
- Oversight and risk management through real-time dashboards focusing on
- Trial management efficiency through comprehensive workflow management for issues, deviations and CAPAs and Milestone task management
- Realise return on investment in just two month with our out of box implementation methodology support by our experts in clinical tool implementation.
- Supports your way of working and your business growth through flexible configuration.
- Minimal IT Effort / Engagement required with 100% web-based solution with integration and reporting tools that is managed by our Ennov technical experts.
eTMF
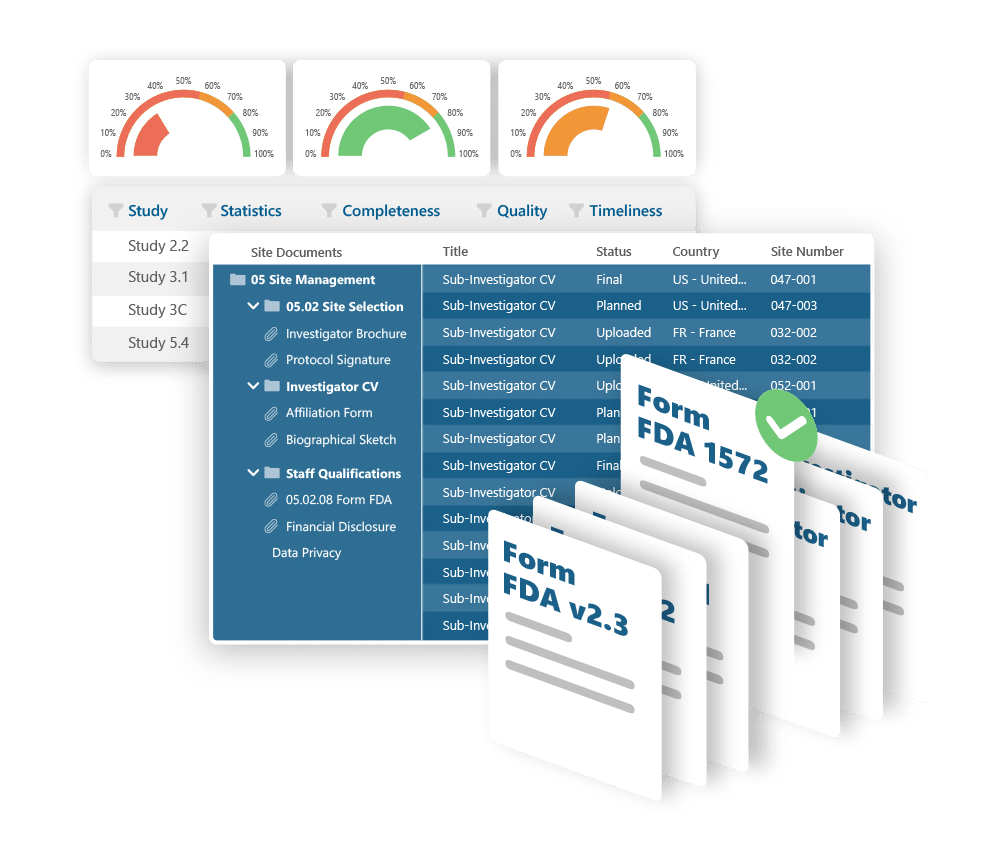
Control your trial documentation. Stay inspection-ready.
Ennov eTMF helps you manage clinical trial documents with confidence: supporting fast startup, full visibility, and effortless compliance. Built around the TMF Reference Model and designed for flexibility, it scales from single studies to global portfolios without complexity.
Key benefits:
- Preloaded with the TMF Reference Model structure
- Metadata-driven document management and easy search
- Real-time visibility into document completeness and QC status
- Supports audits and inspections with traceable, compliant workflows
- Highly configurable without coding or customization
- Secure, scalable, and cost-effective - cloud or on-prem deployment
eTMF Archive
Secure. Compliant. Always inspection-ready.
Ennov TMF Archive ensures long-term preservation of your trial master files in a fully compliant, searchable repository. Designed to meet regulatory expectations for document retention, it keeps your TMF accessible, traceable, and audit-ready for years to come.
Key benefits:
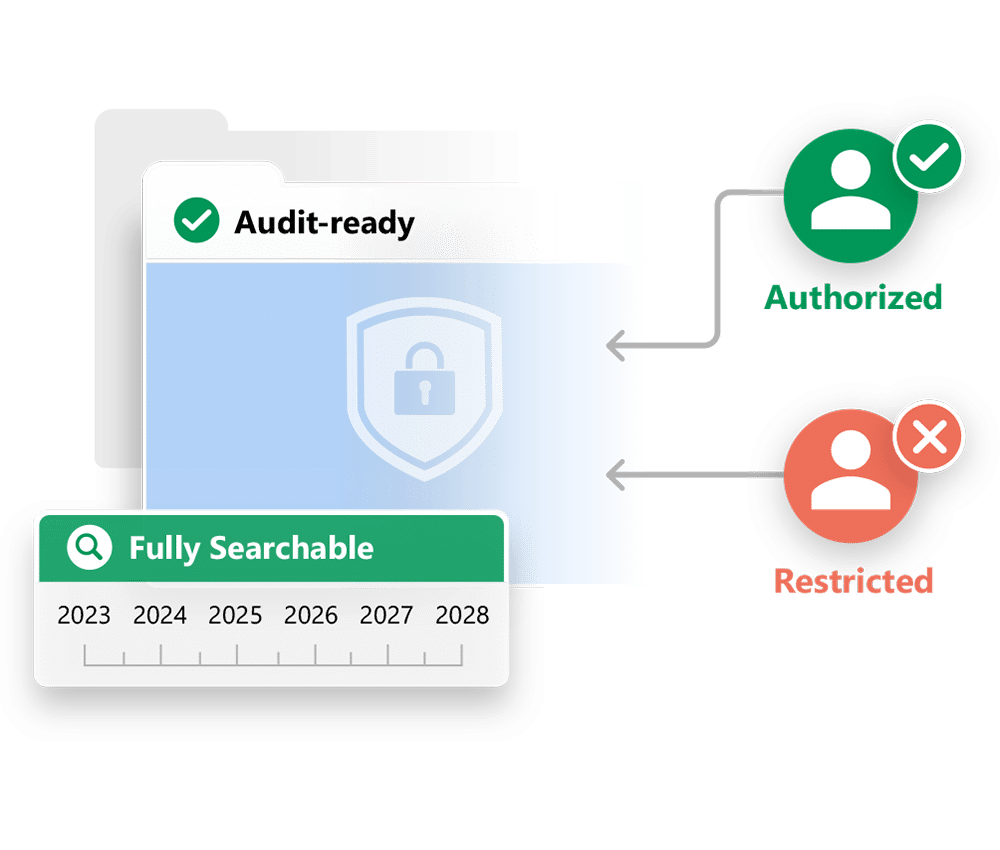
- Long-term, regulation-compliant TMF storage
- Fully searchable with metadata and indexing
- Secure and access-controlled for authorized users only
- Supports inspection-readiness with traceable access logs
- Cloud or on-prem deployment with minimal IT overhead
RTSM
Smarter randomization. Seamless supply management.
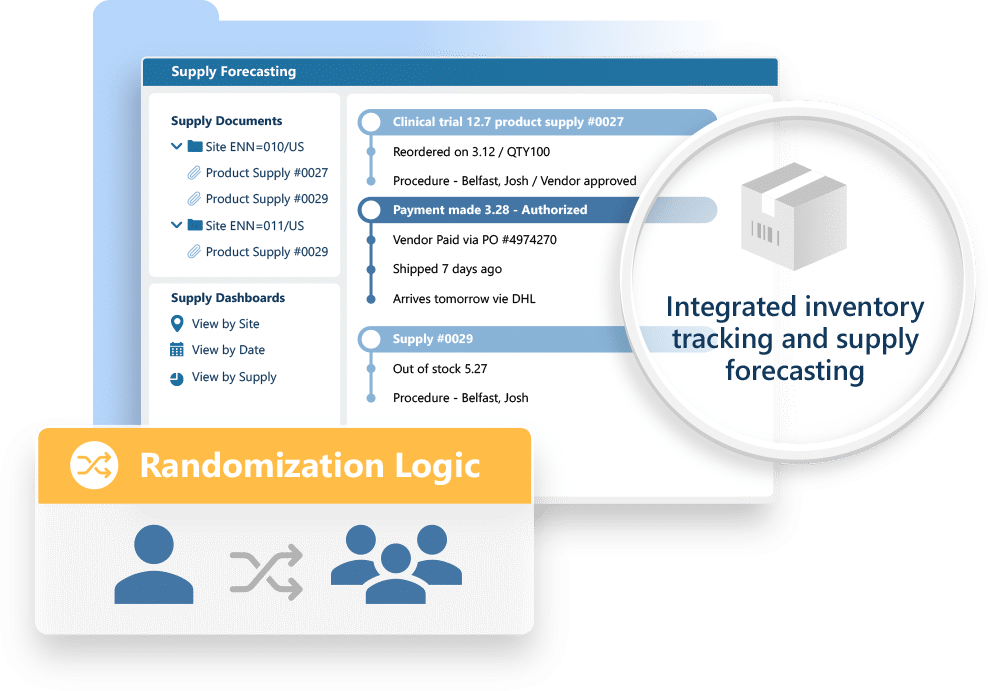
Ennov RTSM simplifies the setup and execution of randomization and trial supply workflows: helping you reduce errors, avoid stock outs, and keep studies moving smoothly across sites and countries.
Key benefits:
- Intuitive, no-code study design and eCRF configuration
- Built-in validations and edit checks to improve data quality
- Seamless integration with Ennov ePRO for direct patient input
- Automated randomization and medical coding (MedDRA, WHO Drug)
- Fast data imports, clean exports, and reliable traceability
- Supports rapid deployment and lower study build cost
