eTMF & eTMF Archive
Control your trial documentation. Stay inspection-ready.
- Preloaded with the TMF Reference Model structure
- Metadata-driven document management and easy search
- Real-time visibility into document completeness and QC status
- Supports audits and inspections with traceable, compliant workflows
- Highly configurable without coding or customization
- Secure, scalable, and cost-effective - cloud or on-prem deployment
The Trial Master File Challenge
Management of essential trial documentation is, indisputably, one of the most time consuming and costly activities associated with conducting a clinical trial. ICH E6 guidance on Good Clinical Practice (GCP) specifies an inventory of over 200 discrete documents that must be managed before, during and after the trial.
Any or all of these documents must be available for audit by the sponsor and for inspection by the regulatory authorities, and considering the massive volumes of documentation involved in the process, the effective management and exchange of study documentation can have a significant impact on the cost and time to complete a clinical trial.
Yet despite the costs in time, effort and money, the trial master file (TMF) is often still managed through a combination of paper and simple shared folders, scattered across many locations in various countries.
Gain Control of Your Clinical Documentation with Ennov eTMF
The eTMF is an electronic trial master file solution to collect and manage essential trial documents in a centralized repository and makes them available to clinical teams, via the internet, from any location at any time. The benefits of using Ennov eTMF include streamlined processes, increased transparency, simplified tracking and enhanced security.
Our eTMF leverages the power of our comprehensive Enterprise Document Management software and Business Process Management software, as they serve as the foundation of the solution. The robust metadata driven document model, highly configurable workflows, powerful searching and flexible views combined with our engaging and intuitive user interface provide all the functionality required to effectively and efficiently manage your clinical trial documentation.

Standards-based but Highly Flexible eTMF Software
The eTMF is a comprehensive solution for managing clinical trial documentation. The document inventory is pre-configured in alignment with the DIA TMF Reference Model and includes all required zones, sections and artifacts.
Ennov eTMF’s metadata-based document model provides the flexibility to adapt this model to your company’s organizational needs. Our intuitive suite of design utilities allow administrators to configure and manage the system without needing IT skills.
Ennov eTMF’s scalability and security enables you to safely manage large volumes of documents – making it the perfect solution for global deployments.
TMF Metrics: The Key to Maintaining TMF Health
Maintaining a high-quality TMF in a near inspection ready state can be a daunting task. Three key metrics are essential to understanding TMF health: completeness, quality and timeliness. Ennov’s dashboards present this information in an easily understandable format and support analysis and drilldown so that the root cause of problems can be identified and addressed. Adjustable thresholds for metrics allow users to understand whether targets are being met at a glance. Our TMF dashboards help organizations to decrease risk, increase efficiency, and improve quality.
Core Capabilities
- Pre-configured document inventory
- Advanced life cycle management
- Flexible rights management
- Automatic PDF rendering
- Scanner integration
- Pre-configured views and tracking dashboards
- Required documents list
Key Features
- Integrated worklist dashboard
- Configurable document types, workflows and views
- Automated email notifications
- Intuitive user interface
- Integated PDF viewer
- 100% web-based
- 21 CFR Part 11 compliant
The Trial Master File Archive Challenge
Sometimes, a sponsor doesn’t need an active TMF. For example, organizations that outsource all of their trials depend on their CRO partners to manage the TMF for ongoing trials. After a trial has been completed, the CRO will usually deliver the TMF to the sponsor, who then has a long-term obligation for the security and accessibility of the TMF. This obligation often extends to 25 years or more.
In this case, the sponsor’s needs are simple, but critical. They must ensure the integrity of the records, limit access to authorized users, and support inspection by health authorities. Full-featured eTMFs may be excessively expensive and complex for this purpose.
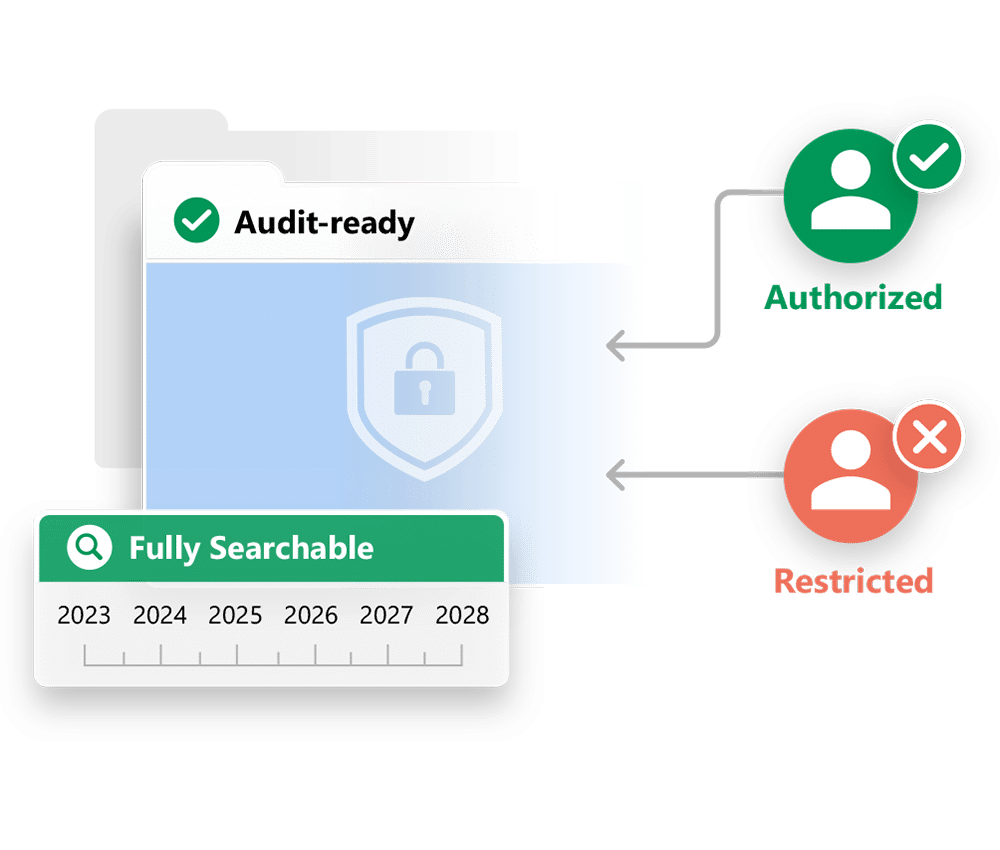
A Simple, Inspectable Archive
The TMF Archive provides a secure archive that is simple to search and navigate. Inspectors can find and review documents quickly and easily by navigating a folder structure based on the TMF Reference Model. They can also access and filter a list view of TMF documents, or use a variety of search tools. They can mark documents as favorites, or access a list of their recently viewed documents.
You can control what an inspector can access based on the scope of their inspection, and view a list of which documents they accessed and in what order.
Documents are easily migrated into TMF Archive. The migration process uses the key data present in any TMF (study, country, site, and document/artifact type) to populate studies. It can also accept and display more advanced information such as document date, expiration date, topic or subject, sub-artifact, or other custom data.
Because the solution is specific to TMF archive needs, validation can be completed in a very short period of time. We provide a complete validation/ user acceptance package to reduce the burden on your users while still providing confidence that the solution is robust enough to meet your needs for many years.
Core Capabilities
- Secure repository
- Flexible rights management – limit inspectors and other users based on need to know
- Folder and Filter View navigation
- Advanced Search
- Favorite and recent document lists
Key Features
- Intuitive user interface
- Easy management of users (internal and inspector)
- Streamlined migration of documents into archive
- 100% web-based
- 21 CFR Part 11 compliant
Efficiently & Securely Capture & Manage Clinical Trial Information
The Ennov Clinical suite consists of Clinical Data Management applications as well as Clinical Trial Management applications that are available for deployment in the cloud or on premises.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
