Complete Audit and Corrective/Preventive Action Management Solution
Quality Document and Process Management
Business Problem
Centralized Audit Management
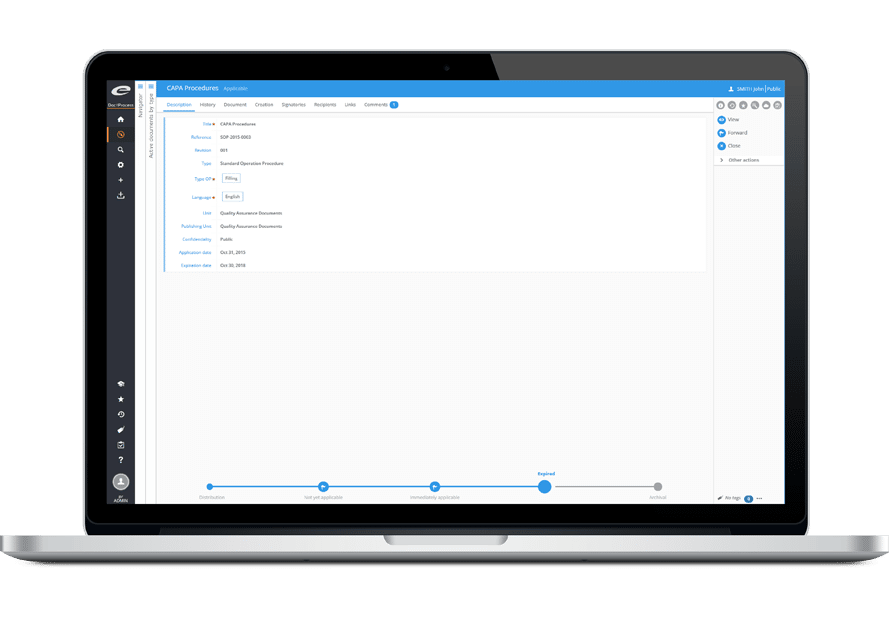
An ISO 9001 certification process must include internal audits at regular intervals, to ensure at that procedures are correctly applied and to validate the effectiveness of the Quality Management system. Ennov improves this efficiency by automating administrative tasks associated with managing audits.
The implementation of internal audits is complex: matrix organization (multi-site, multi-product), the variety of skills required and the number of applicable standards.
The efficient management of audits requires the use of software to optimize the time spent, standardize evaluation methods and effectively monitor corrective and preventive actions.
Ennov offers a complete solution for managing audits and corrective and preventive actions.
Each audit is managed from start to finish:
- Team composition
- Planning
- Program definition
- Recording remarks and deviations
- Report generation and validation
- Follow-up of corrective and preventive actions
- Control of resolution times
- Planning follow-up audits
In conjunction with the procedure documents managed in Ennov Doc, the Ennov Process workflow software drastically reduces the administrative burden on auditors – enabling them to concentrate on their business, optimize their planning and capitalize on the knowledge acquired during audits. From a web interface, you have, according to your authorization, an overview of the activity relating to the audits, summarizing:
- Audits and actions planned, in progress or completed
- Reports produced by site or by field
- Remarks and deviations classified by severity level or expected resolution date
- The schedule of tasks for each auditor or expert.
Proposed Solution
Quality Audit Planning and Resource Optimization
Ennov software automates audit planning. The calendar is displayed by site, by domain or by auditor.
This facilitates the establishment of audit teams. When a new assignment is being prepared, you can immediately see which auditors and experts are available (depending on the skills required).
Ennov also manages periodic audits, which are automatically initiated based on rules you define.
Following each audit, the people involved enter the time spent on site (in hours), writing documents and administrative follow-up, travelling (multi-site companies). This makes it possible to gather precise statistics on the workload per profile and thus to formulate realistic workload plans going forward.
Automatic Audit Report Generation
As the assignment progresses, the auditor conducts one or more questionnaires, in conjunction with the applicable repositories managed in the DMS software. The audit report is automatically generated: the information entered during the audit is automatically imported into a predefined office model (for each type of audit). The document can remain editable if adjustments are necessary.
This report is validated in accordance with ISO 9001 requirements for electronic signatures, then distributed via Ennov to all stakeholders concerned (a notification is sent by email).
The audit report is stored in the quality software, and remains available for those with the necessary clearance. The history of the reports produced for a given site or a particular domain is thus accessible in a few clicks.
Deviation Traceability and Follow-up Actions
Notes or deviations are recorded for each quality audit. The number and severity will determine the overall assessment and the decision to initiate corrective or preventive actions.
These corrective and preventive actions are managed in the Quality Management software, each following its own workflow according to the rules defined in the system (responsibilities, deadlines, efficiency evaluation).
Ennov allows you to search for actions already created according to all the criteria you wish: urgency level, due date, site, product line and the ability to navigate between the actions and the audits which originated them (bi-directionally).
The QMS functionality provided by Ennov are used to effectively monitor actions and manage alerts in case of delays. The delegation function is also useful to ensure that a person’s absence does not delay the processing of an urgent action.
Audit Management Features
- Complete management of the audit lifecycle: Preparation, planning, execution, validation, management of the actions associated with each deviation
- Establishment of audit teams for each planned engagement, with task and schedule management
- Record remarks and deviations detected for each audit
- Automatically generated and retained audit documents
- Launch of corrective and preventive action plans in connection with audits
- Evaluation of the effectiveness of corrective and preventive actions
- Global vision of the audits carried out by site, by auditor, by theme
- Automatically generated dashboards and monitoring charts: Average score per audit, auditor occupancy rate, number of deviations per site
Why Choose Ennov
Hundreds of corporate customers trust Ennov
Over 15 years of experience providing Quality solutions
150+ life science customers, many more in other industries
Modern architecture and interface
100% web-based. Highly scalable. User-centric design
Our commitment to your success
Very high customer satisfaction. 98.5% of projects delivered on time and within budgets.
Hundreds of corporate customers trust Ennov
Available as cloud-based or on premises deployment
You can switch between deployment options at any time.
We make you autonomous
System configuration and management require no IT skills.
Improved security and optimized performance
Data is hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
