Ennov PV
Case Intake & Management
Capture confidently. Configurable workflows. Stay compliant.
- Accurately capture and manage both human and veterinary PV cases
- Import adverse event data or enter directly using flexible templates
- Fully configurable workflows to support end-to-end case processing
- Seamlessly integrates into existing pharmacovigilance operations
- Trusted by pharma, CROs, service providers, and regulators
- Scalable, compliant, and designed to fit your business processes
Case Intake & Management
Efficient Data Entry of Pharmacovigilance Cases
This web based application revolutionizes pharmacovigilance case management. The interface has been optimized to meet the specific needs of those users engaged in receiving reports, entering data, and handling case follow-ups. Many functions have been developed in response to actual customer feedback to promote efficiency and improve the end user experience. Traditional PV systems have been focused on the needs of the data reviewers and users responsible for trending and analysis.
Data can be captured in structured format using standard vocabulary lists, with very little end user training. Cases can be captured in local language and automatically translated at the time of import into the PV system. Additionally, the solution can be deployed as a lightweight PDF file or as a dynamic browser-based web form optimized for smartphones and tablets.
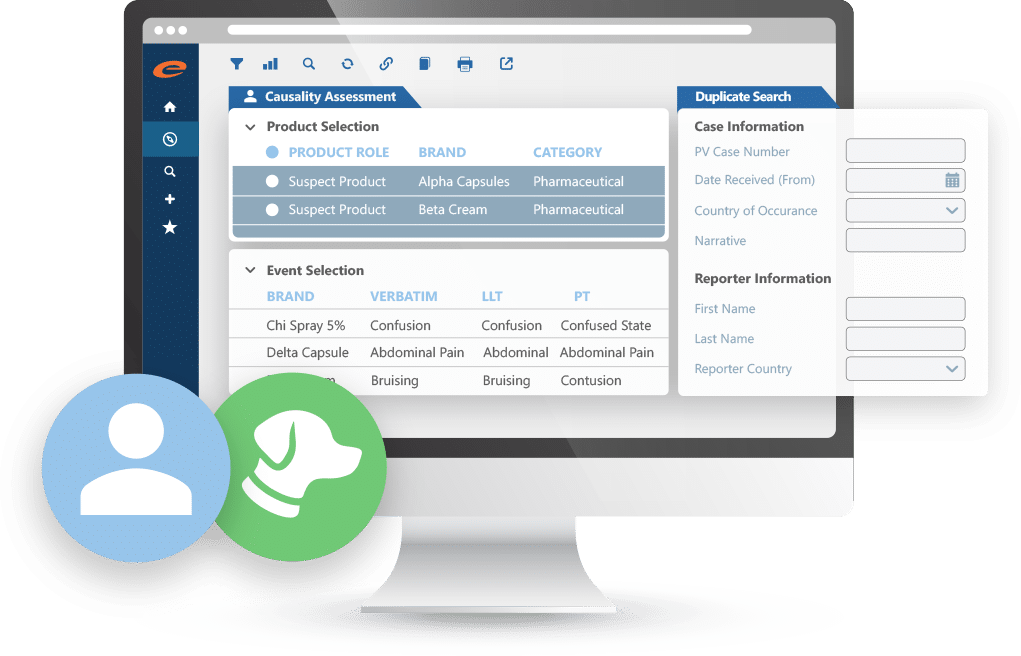
- ICH E2B R3 compliant PV database
- Simplified MedDRA coding
- Paper and electronic reporting formats
- Workflow driven with powerful querying and analysis tools
- Optimized user interface for efficient data entry
Core Capabilities
- Optimized case capture interface
- Search engine style querying
- Configurable data section and field layouts
- Searchable vocabulary lists
- Rapid coding tool
- Drag-and-drop palette for frequently used data
- Speech to text for narratives
- Social style case management tools
Key Features
- Data entry interface is configured to mirror call flow
- Dramatically speeds up data entry
- Modern and intuitive layout
- Optimized for call center operations
- Reduces training overhead and accelerates on-boarding
- Seamless connectivity to PV-Works
- 100% web-based
- 21 CFR Part 11 compliant
An End-To-End Solution for Collecting, Reporting and Analyzing Human and Vet PV Data
The Ennov Pharmacovigilance solution keep the collection, management, assessment, and reporting of human or veterinary adverse events in one unified database while also providing advanced signal detection and PV data analysis tools.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
