Ennov Compliance Platform
A Unified Approach to Regulated Content and Data Management
Ennov’s unified compliance platform is a highly configurable software architecture that combines business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed.
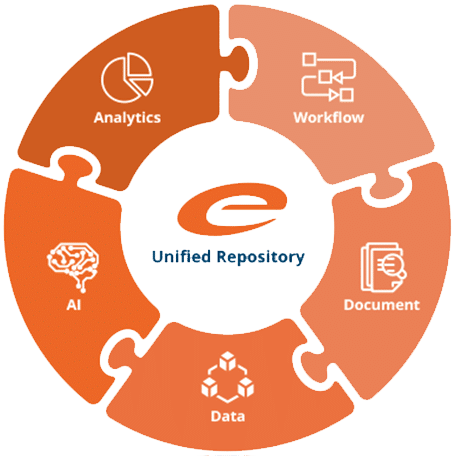
Unified Platform for Regulated Content and Information Management
Ennov’s unified compliance platform is a highly configurable software architecture that combines a common and engaging user experience (UX) with business process management, document management, data/forms management, learning management and business intelligence to form the foundation on which our applications are constructed. As a result, our applications are natively connected and do not require integrations or interfaces to facilitate interoperability. A common, harmonized and configurable data model eliminates redundancy and ensures data accuracy and consistency across all applications.
Benefits
Because all applications share a common UX, users can focus on completing their assigned work and not on mastering the nuances of multiple specialized systems. Common activities such as navigation, searching, item creation and task acquisition are performed identically – regardless of context. This unified and ubiquitous approach results in higher user adoption rates, lower training requirements and overall increased productivity.
Why it Matters
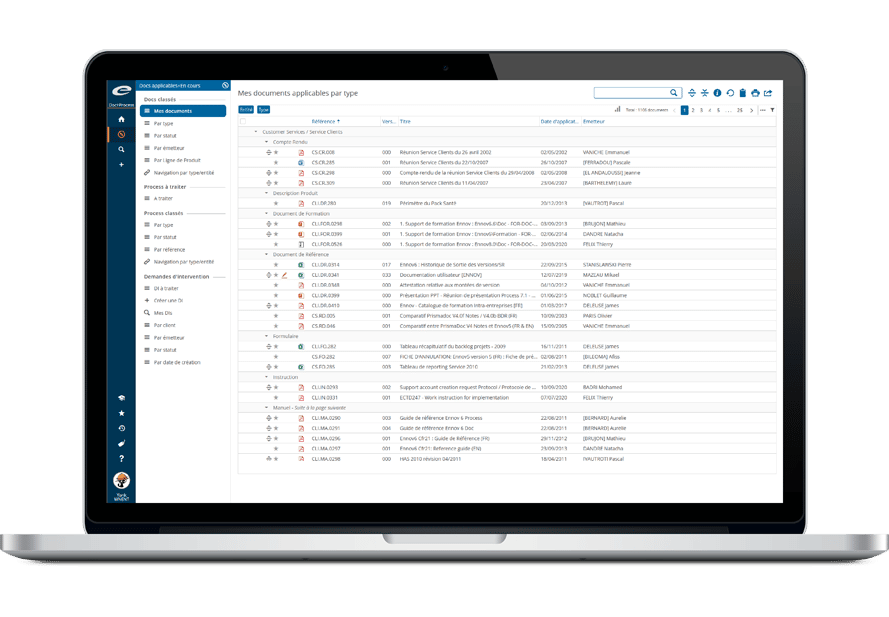
Ennov Document
Unified Access to All Documents for Improved Efficiency
The Ennov platform contains a single data repository that can manage the entire document lifecycle: creation, collaborative authoring, approval, distribution, revision/cancellation, archival. When a document is revised, the new version is created in draft status; it will become applicable after being signed by all approvers, the previous version is then automatically archived. Ennov’s metadata-based document model is flexible, easy to configure and adaptable to your company’s organizational needs. Ennov’s highly scalable architecture supports the management of large volumes of documents and users – making it the perfect solution for global deployments.
Ennov Data
Facilitate Data Management with a Single Repository
The Ennov platform relies on a unified and unique data repository, shared by all modules of the software. Data is stored only once and made available to all services and software that need to use it. This guarantees data homogeneity, as it is no longer necessary to manage silos and reconcile data from multiple sources. All of this contributes to increased confidence in the data within the organization, which has a direct impact on operational performance.
Ennov Workflow
Manage and Track Workflows with Business Process Management Software
Ennov Workflow allows you to automate your business process workflows to enforce the event sequence, workflow participants and business rules defined by your standard operating procedures. Processes that deviate from the defined path or remain stalled for months become a thing of the past.
Ennov Workflow has a flexible, contemporary user-friendly interface and is fully configurable to meet the needs of diverse organizational structures and their associated processes. The process form associated with each workflow retains the information needed at each process step. Each workflow participant has the information needed to complete their task in the proper context. Email notifications and escalations ensure that no process step becomes stalled. Now anyone can follow any activity, track pending or completed tasks and monitor participant activity to eliminate bottlenecks and avoid delays.
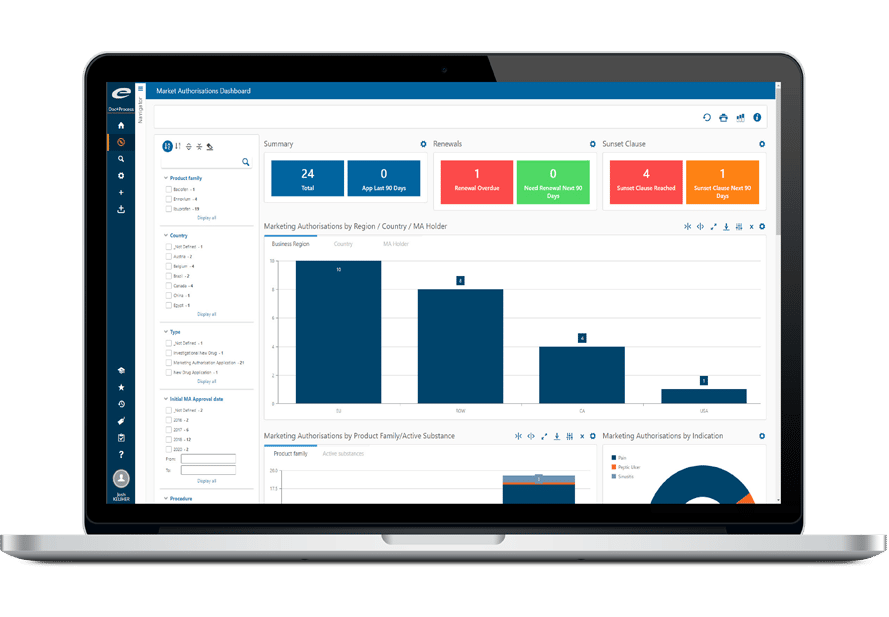
Ennov Analytics
Optimize performance using our innovative Business Intelligence software
Ennov Analytics includes powerful yet easy-to-use reporting and visualization capabilities that capture the metrics required to produce key performance indicators from the data stored within Ennov applications. Reports and dashboards are managed natively within the platform and as such, they are easily accessed by Ennov users through the same common interface.
Ennov Analytics feature provides comprehensive access to the information managed in the different Ennov applications. As well, Ennov Analytics includes a feature-rich visual display library with a wide variety of graphical components to help you design the most attractive reports and dashboards. With Ennov Analytics, the focus is on strengthening your strategic decision-making while ensuring the information being managed on a day-to-day basis is presented in a more meaningful and useful manner.