Analyst Report
Still stitching together e-clinical tools?
The industry is moving on.
View the report
Point solutions are out. Unified platforms are in, and they’re reshaping cost, compliance, and data strategy.
Why It Matters?
- Disconnected tools are slowing down trials, increasing risk, and complicating compliance.
- This Gartner report explains why integrated platforms are the future, and how to evaluate the right vendor.
- Ennov is listed as a Representative Vendor for E-Clinical Systems
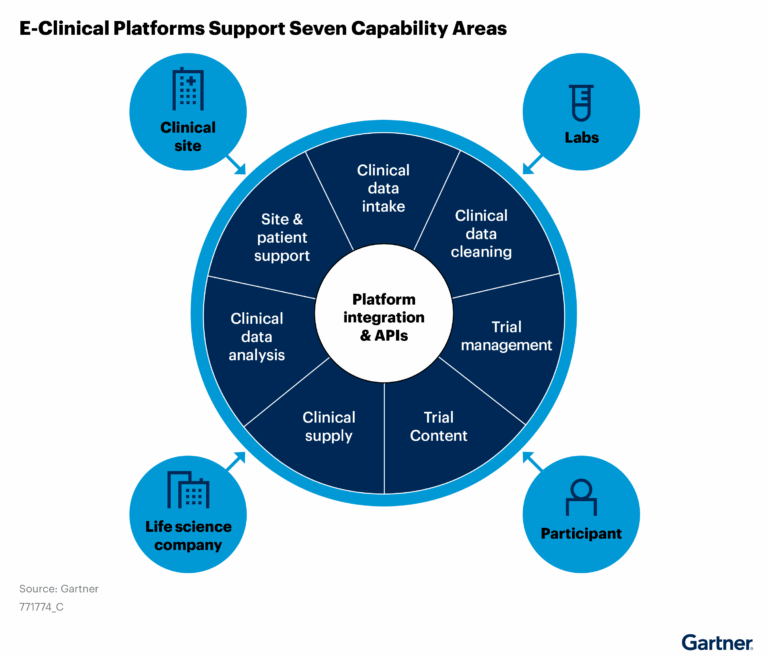
“These trends fuel a shift in buying behavior away from e-clinical point solutions to integrated e-clinical platforms that offer the efficiency of an aggregate solution without sacrificing the business-unit-specific capabilities of point solutions.”
View the report
Ennov may use your contact information to provide updates and special offers about Ennov products and services.
Market Guide for Life Science E-Clinical Systems, By Jeff Smith. 25 June 2024
GARTNER is a registered trademark and service mark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and is used herein with permission. All rights reserved.
Gartner does not endorse any vendor, product or service depicted in its research publications, and does not advise technology users to select only those vendors with the highest ratings or other designation. Gartner research publications consist of the opinions of Gartner’s research organization and should not be construed as statements of fact. Gartner disclaims all warranties, expressed or implied, with respect to this research, including any warranties of merchantability or fitness for a particular purpose.
This graphic was published by Gartner, Inc. as part of a larger research document and should be evaluated in the context of the entire document. The Gartner document is available upon request from Ennov.
