IDMP Content Hub
With IDMP deadlines approaching fast, here is everything you need to know, in one place.
- Mid-2026: Critical Medicines PMS Enrichment
- By 2027: Full Portfolio Enrichment
Discover Our IDMP Resources
IDMP Timelines
[Downloadable PDF timeline]

The European Shortage Monitoring Platform
[Blog Article]

IDMP Starter Pack
[Practical Guide]

The IDMP Countdown
[Podcast]

Mind the PMS Data Gap
[Podcast]

Best Practices - Master Data Management
[White Paper]
IDMP Standalone Agnostic Approach: Challenges and Achievements
[GPRAS 2025 Conference Session]
Ennov Offers the Only RIM Agnostic Solution for Automated PMS Enrichment
Become IDMP-ready without changing your RIM system or MDM.

- IDMP EASI provides an instant, live comparison report of your RIM data versus PMS for straightforward enrichment. It can also send a validated FHIR message to update PMS.
- It is designed to cut the enrichment time from hours to minutes.
- Everything you need to be ready before the June and December deadlines, without changing your established RIM processes.
- Fast, automated, and built for scale.
Go from hours per product to minutes when preparing for IDMP.
Be ready for the June & December 2026 deadlines.
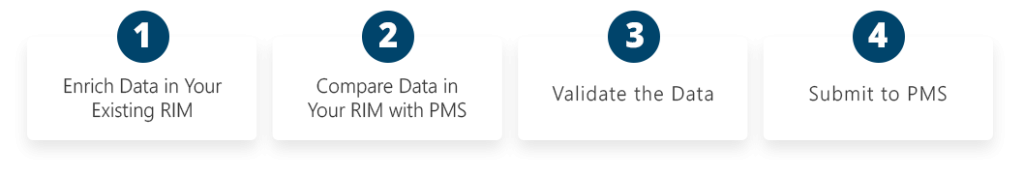

What IDMP EASI is?
- A fast, agnostic readiness layer
- Works with any RIM or data source
- Automates PMS comparison, IDMP validation, and FHIR preparation
- Sends data directly to PMS at the click of a button
- The only portfolio-scale solution on the market
What IDMP EASI is not?
- Not a RIM
- Not a migration
- Not a replacement for existing systems
Use Our IDMP EASI Calculator to Estimate Your PMS Workload
It’s free, and only takes 5 clicks to discover how much time you can save with our IDMP EASI tool.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
Frequently asked questions on IDMP
What are the latest IDMP deadlines by EMA for 2026?
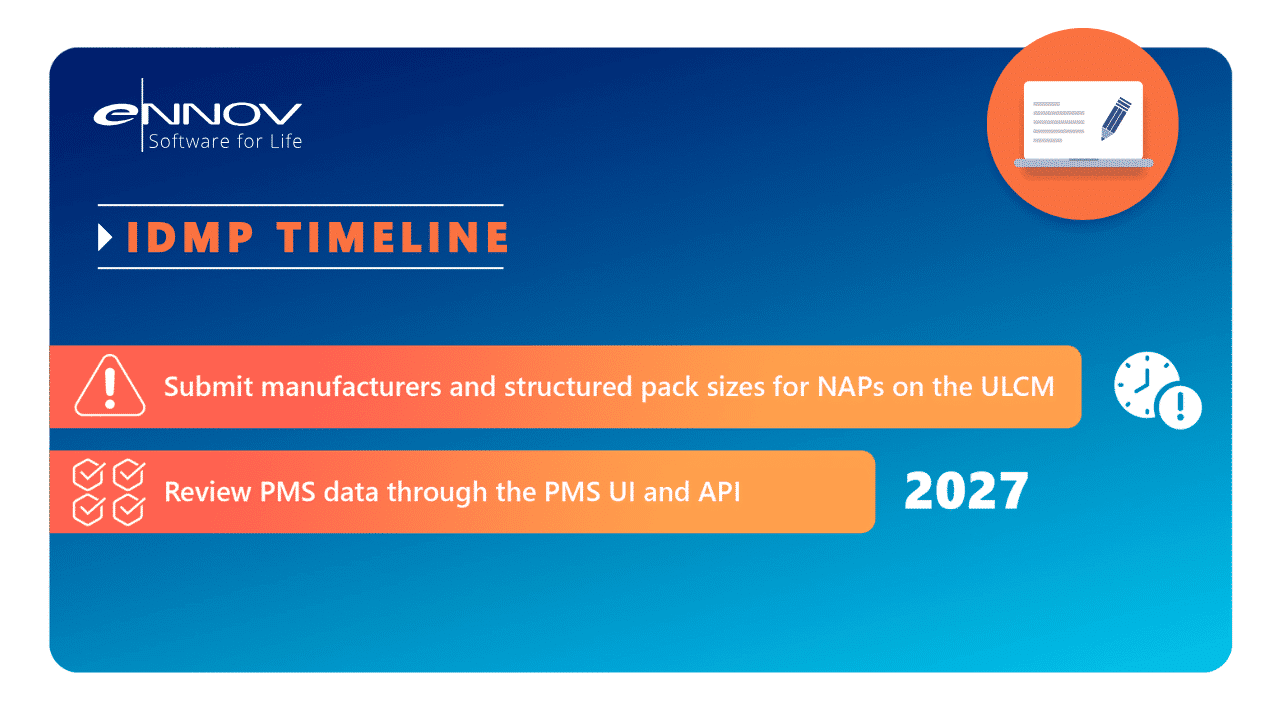
The EMA has introduced phased deadlines for submitting structured product data into the Product Management Service (PMS) as part of IDMP compliance. The 2026 milestones focus primarily on non-centrally authorised products (non-CAPs):
June 2026:
Marketing authorisation holders must submit structured manufacturer data and pack size information for non-CAPs that appear in the Union List of Critical Medicines (ULCM).December 2026:
All remaining non-CAPs must have their structured manufacturer data submitted to PMS.
These deadlines are part of the broader transition from XEVMPD to full ISO IDMP data standards and are designed to support improved data quality, interoperability, and regulatory decision-making across Europe.
Where can I find the EMA IDMP implementation guide?
The EMA IDMP implementation guide is available on the European Medicines Agency website within the SPOR (Substance, Product, Organisation, Referential) section, under the documentation for the Product Management Service (PMS).
What is the June 2026 deadline for critical medicines?
The June 2026 deadline requires marketing authorisation holders to submit structured manufacturer and pack-size data into EMA’s Product Management Service (PMS) for all non-centrally authorised products (non-CAPs) that appear on the Union List of Critical Medicines (ULCM).
Where can I find the list of critical medicines for IDMP compliance?
The list of critical medicines used for IDMP compliance comes from the Union List of Critical Medicines (ULCM), published by the European Medicines Agency and the European Commission.
You can find it on the EMA website, within the section on medicine shortages and critical medicines monitoring. Link is here.
What IDMP software are available today on the market?
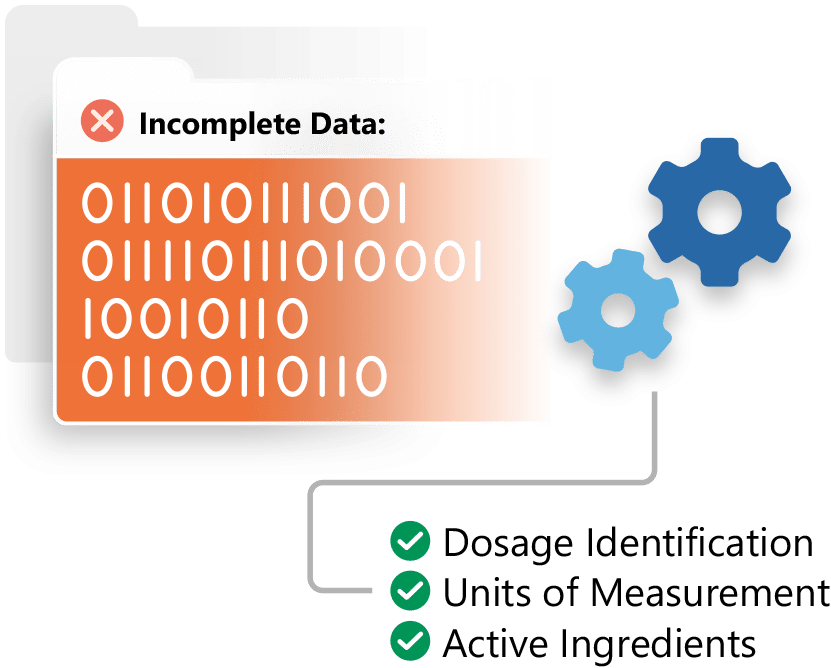
Most life sciences organisations use a combination of Regulatory Information Management (RIM) systems and specialised data-management tools to prepare, validate, and submit the structured product data required under EMA’s IDMP standards. These solutions typically support data extraction, enrichment, validation against ISO IDMP rules, and submission to the Product Management Service (PMS).
Ennov offers a comprehensive approach to IDMP compliance.
Our unified platform includes robust regulatory data management capabilities, and our EASI solution provides an additional readiness layer designed specifically for IDMP. EASI helps organisations assess the completeness of their product data, automate enrichment at scale, and prepare PMS-aligned datasets, regardless of the underlying RIM system. This allows teams to reduce manual work, improve data quality, and accelerate IDMP compliance across large product portfolios.
Is IDMP a one off activity?
No. IDMP is an ongoing process, not a one-time project.
Once organisations submit their initial data to EMA’s Product Management Service (PMS), they must continue to maintain, update, and validate that data throughout the entire product lifecycle. Any regulatory change, variation, new pack, reformulation, or update to referential data can trigger new IDMP obligations.
Because product information evolves constantly, IDMP compliance requires continuous data quality management, repeatable validation cycles, and operational processes that can keep pace as guidance and requirements evolve.
What's the deadline for the rest of the portfolio?
For products not on the Union List of Critical Medicines, EMA has set a later deadline.
Marketing authorisation holders must submit structured manufacturer data for all remaining non-centrally authorised products (non-CAPs) by December 2026. Additional data elements, such as pack-size information for the broader portfolio, are required by June 2027.
These phased deadlines allow organisations more time to prepare their full portfolios, but data readiness work should begin early, as large product sets require significant enrichment, validation, and iterative alignment with evolving PMS expectations.
How is IDMP different than xEVMPD?
IDMP and xEVMPD both support the exchange of regulatory product information in Europe, but they differ significantly in scope and structure.
xEVMPD focuses on a defined set of product data submitted in a single format for pharmacovigilance purposes. It is largely document-driven and relies on less granular data structures.
IDMP introduces a far more detailed, structured, and standardised data model based on international ISO standards. It requires medicinal product information to be broken down into discrete, validated data elements that can be continuously updated and exchanged through EMA’s Product Management Service (PMS). This creates a more accurate, interoperable, and lifecycle-oriented view of product data across the industry.
For a deeper comparison, you can read our full article here:
https://en.ennov.com/blog/regulatory-blog/idmp-xevmpd-compliance/
What is the relationship between master data management (MDM) and IDMP?
Master data management (MDM) provides the foundation for successful IDMP compliance. IDMP requires product information to be complete, consistent, structured, and continuously maintained across systems. MDM supports this by establishing a single source of truth for key data elements such as product names, ingredients, organisations, manufacturing sites, and pack details.
A strong MDM approach helps organisations avoid duplication, reduce manual corrections, and ensure that updates flow reliably into EMA’s Product Management Service (PMS). In practice, MDM and IDMP reinforce each other: MDM improves data quality and governance, while IDMP provides the standards and structure needed to maintain that data across the product lifecycle.
What is the role of HL7 FHIR in IDMP data exchange?
HL7 FHIR provides the technical framework for exchanging IDMP-compliant data between regulatory systems. While IDMP defines what data must be represented (substances, products, organisations, referentials, and their attributes), FHIR defines how that data is structured, packaged, and transmitted.
EMA uses FHIR to support interoperability across SPOR services, including the Product Management Service (PMS). This ensures that product information is exchanged in a consistent, machine-readable format that can be validated, updated, and reused across regulatory processes.
FHIR is therefore a key enabler of IDMP: it turns the ISO standards into a practical, scalable way for authorities and industry to exchange high-quality medicinal product data.
How can we help you?
Fill out the form and we'll be in touch.