Ennov Compliance platform
Not Just a Platform. A Unified Ecosystem for Life Sciences.
Replace siloed systems with one unified ecosystem built for collaboration, compliance, and scale.
- One login across Clinical, Quality, Regulatory, and Pharmacovigilance
- One data repository where all product and operational data lives
- One audit trail for end-to-end regulatory compliance
- One vendor who understands all your needs
One Ecosystem. Every Function. Zero Compromises.
Unlike vendors who bundle separate apps behind a shared interface, Ennov is unified at the core: one data model, one repository, no integrations required.
It’s more than a platform. It’s a single ecosystem built from the ground up for life sciences.
How the Ennov Ecosystem Works
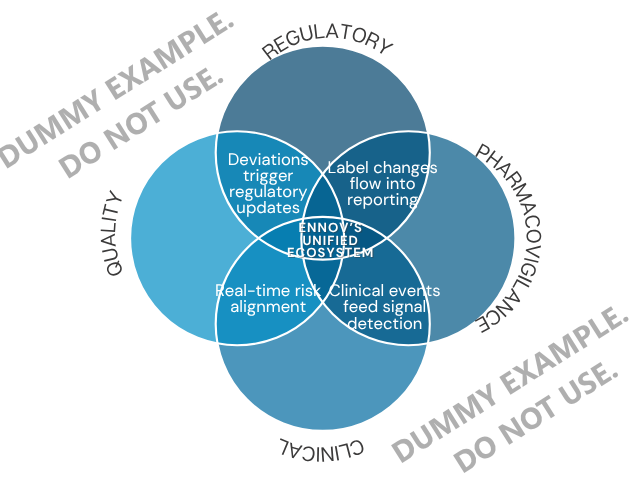
Complete Solutions, Built to Work Together
Each suite is powerful on its own. Together, they share data to form a unified ecosystem—no connectors, no silos.
CLINICAL
eTMF, CTMS, ePRO, Remote Monitoring
- Uses shared site and product data from Quality
- Feeds directly into Regulatory workflows
QUALITY
Audits, CAPAs, Document Control, Training
- Shares master data with Regulatory and Clinical
- Drives faster, inspection-ready compliance
REGULATORY
RIM, IDMP, Submissions, Publishing
- Pulls directly from Quality records
- Connects to Clinical for real-time submission updates
PHARMACOVIGILANCE
Case Processing, Signal Detection, Reporting
- Auto-updates from Regulatory label changes
- Links to Quality for manufacturing-related safety issues
Start Anywhere, Expand Everywhere
- Begin with your biggest pain point (most customers start with Quality or Regulatory)
- Add new functions without disrupting existing workflows
- Leverage existing data and user training as you expand
Global Scale, Local Compliance
- Single platform supporting FDA, EMA, PMDA, and other regulatory requirements
- Consistent processes across geographies with local regulatory flexibility
- One vendor relationship, multiple regional capabilities
"We went from five disconnected systems to one living ecosystem without losing any functionality. Actually, we gained capabilities we never had before because everything finally works together naturally."
— IT Director,Global Pharmaceutical Company
"During our last audit, we could show real-time connections between quality issues and regulatory responses. The inspector said our ecosystem approach was 'exactly what modern compliance should look like."
— VP Quality Assurance, Medical Device Manufacturer
"Growing our business used to mean new systems, new training, new integrations. With Ennov's ecosystem, it just means new users in the same natural environment"
— Head of Quality & Regulatory,Specialty Pharma
Ecosystem Architecture Built for Life Sciences
- Single data repository: No data duplication or synchronization issues
- Natural workflows: Cross-functional processes flow organically without API dependencies
- Simplified validation: One qualification cycle for the entire ecosystem
- Role-based access: Appropriate visibility across all functions
- Real-time adaptation: Instant updates flow naturally across all connected processes
- Fully compliant: 21 CFR Part 11, EU GMP Annex 11, ISO standards
Ready to Experience a True Ecosystem?
Fill out the form below to request a demo.
