Ennov regulatory suite
Dossier
Submission Publishing Software
- Create, manage and publish Regulatory submissions
- Publish to any output format
- Robust hyperlinking and bookmarking
- Built in validator ensures compliant submissions
- 100% web-based, ideal for global deployments
The Submission Publishing Challenge
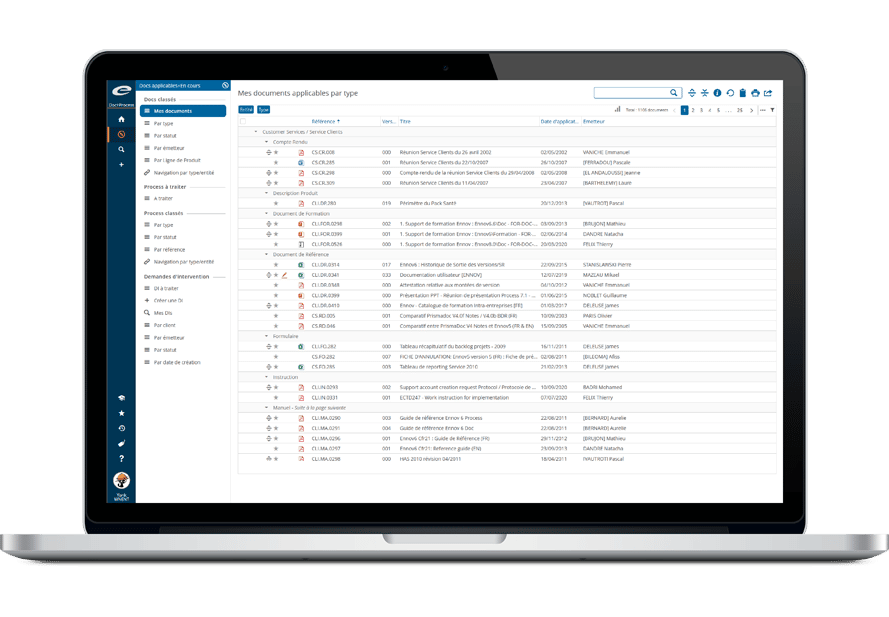
The efficient production of compliant regulatory submissions is the goal of every regulatory operation. Having a single publishing solution that can effectively produce a variety of submission output formats provides a distinct advantage in terms of flexibility, training and total cost of ownership.
Ennov Dossier is a complete and scalable dossier management and submission publishing solution that is suitable for regulatory operations of all sizes. Ennov Dossier produces output that is compliant with all current health authority requirements:
- CTD (Common Technical Document) for paper publications
- eCTD and NeeS (Non eCTD electronic submission) for electronic publications
- vNeeS for the veterinary sector
- eCopy for medical devices
Ennov Dossier is comprehensive and meets the most demanding requirements for regulatory submissions while still being intuitive and easy to use.
Efficient and Easy to Use
Ennov Dossier provides the ability to build, manage, publish, validate and archive regulatory dossiers using the native capabilities found within Ennov Doc.
This eliminates the fragmented and inefficient processes of locating, copying and uploading the documents that you need for your regulatory submissions – providing a harmonized and seamless dossier publishing solution.
A simple drag-and-drop interface allows publishers to link documents into submission assemblies quickly and easily.
Improve Productivity with our Integrated EDMS
Ennov Dossier, when combined with Ennov Doc, provides the ability to manage your regulatory dossiers using all of the robust functionality of our comprehensive EDMS.
Automated dossier life cycles, workflows and notifications eliminate fragmented manual processes and increase productivity.
Ennov’s unique metadata-based navigation also helps improve productivity by allowing users to quickly locate dossiers using their properties rather than a storage location in a filing hierarchy.
Compliant Submissions Every Time
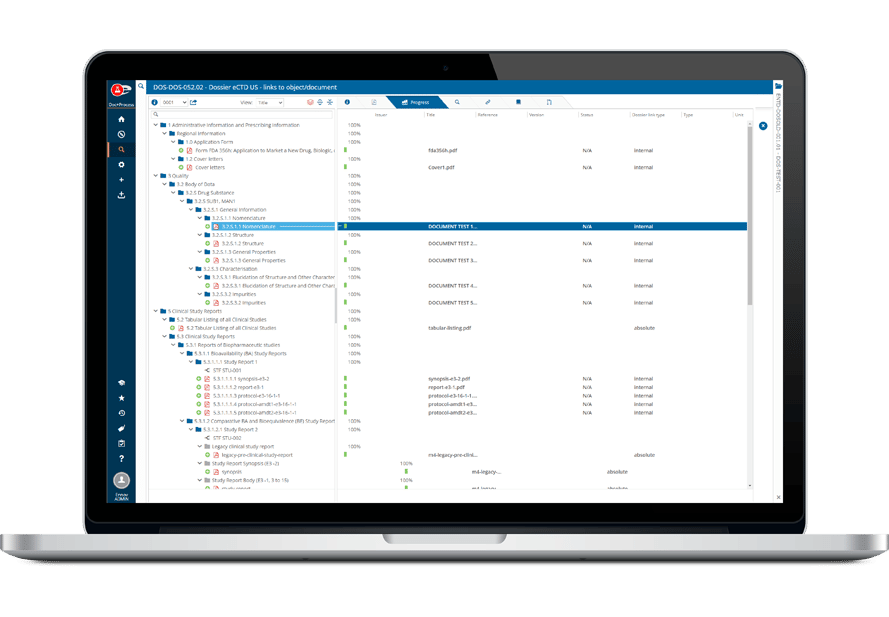
Submission assembly templates are provided for the regions that accept eCTD submissions (e.g. US, EU, GCC, Canada, Swissmedic, TGA) as well as for other non-eCTD formats and can be modified to meet a client’s specific requirements. Ennov provides regular updates to these templates as the regulatory guidance changes.
Ennov Dossier also provides the ability to create Tables of Contents, hyperlinks, bookmarks and other navigation aides to assist in the review of the dossier.
During publishing all the necessary components for a compliant submission, including the required ICH and regional XML files, correctly named leaf files and folder structures are created.
Core Capabilities
- CTD, eCTD, NeeS, VNeeS and eCopy support
- Dossier life cycle management
- eCTD sequence and metadata management
- Robust hyperlinking and bookmarking
- Integrated eCTD validator
- Built-in submission assembly templates
- Full text and metadata based searching
Key Features
- Intrinsically connected with Ennov Doc
- Intuitive drag-and-drop user interface
- Compatible with any WebDAV compliant repository
- Automatic compliant PDF rendering
- 100% web-based
Aguettant Case Study
Cyrille JEUNE, Regulatory Affairs Systems Manager
Ennov Regulatory
World-Class Regulatory Content And Information Management
The Ennov Regulatory suite combines the power and flexibility of Ennov Doc, Ennov Dossier and Ennov Process to support the entire regulatory product lifecycle from the early planning of registration targets through product retirement. The Ennov Regulatory suite is an invaluable tool for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov ?
Over 500,000 users trust Ennov
Over 25 years of experience providing software solutions for Life Sciences
450+ Life Science customers, many more in other industries.
Modern architecture and interface
100% web-based. Highly scalable. User-centric design.
Our commitment to your success
Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Available as cloud-based or on-premises deployment
You can switch between deployment options at any time.
We make you autonomous
System configuration and management require no IT skills.
Improved security and optimized performance
Data is hosted locally for total flexibility. Single-tenancy minimizes business interruptions.

Cloud-based or On Premises

Multi-Platform