IDMP Data Management Solution
Prepare your organization for the emerging ISO standards and health authority requirements with Ennov IDMP
- Simplified IDMP management
- Integration with the EMA’s PMS increased data quality
- Ennov IDMP services
- Works with any RIM system to ensure IDMP compliance
Simplify IDMP Management
The ISO Identification of Medicinal Products (IDMP) standards are a set of common, global standards for data elements, formats, and terminology to identify and exchange information about medicinal products.
The introduction and progressive implementation of new IDMP standards means that organizations will need to capture and manage significantly more regulatory data than ever before, which represents a significant challenge to the industry as a whole.
Effective data management involves significant effort, is time-consuming and costly. Ennov IDMP and our IDMP services simplify the process of maintaining your IDMP data while transitioning to the new standard.
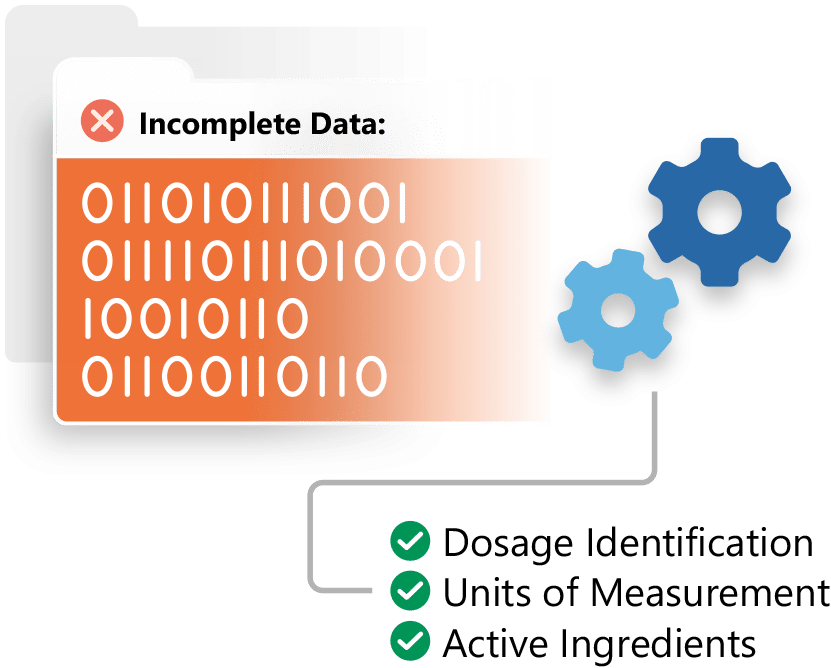
Ensure IDMP Data Quality
Ennov IDMP is a quality controlled solution for data management in support of the new IDMP standard.
Leverage your existing data to get a jump start on IDMP. Inventory, identify, cleanse and integrate your data, making your organization IDMP ready.
Mine and extract information contained in your regulatory documentation and other enterprise systems to ensure completeness.
No Time to Overhaul Your RIM? You Don’t Need To.
Stay compliant with our plug-and-play IDMP solution.
Connected to the Future
Ennov RIM is IDMP ready. We currently support the EMA SPOR Referentials lists (controlled vocabularies) within the system and flag each data element with an “IDMP Term” indicator.
As the SPOR data services specifications evolve, they will be systematically incorporated into the solution – taking full advantage of the Ennov Platform’s flexibility and configurability.
Ennov IDMP is a key component in our comprehensive Regulatory solution suite. In combination with Ennov Doc, Ennov RIM, Ennov Dossier, Ennov Report and our REST API, Ennov has the solution today to whatever challenges tomorrow may bring.
Prepare for IDMP Compliance
Ennov recognizes that compliance with ISO IDMP represents a huge challenge for companies and addressing this challenge places an additional burden on their Regulatory resources.
In an effort to help the industry effectively and efficiently meet the requirements of this new standard, Ennov offers an IDMP Readiness Assessment service as a crucial first step in preparing for IDMP submissions.
Performing the assessment results in a documented evaluation that identifies the various data sources (both structured and unstructured) and provides an accounting of the gaps that will need to be addressed to ensure IDMP compliance.
Core Capabilities
- Use of available controlled vocabularies
- Assistance with data extract, cleansing and migration
- Pragmatic IDMP data management
- Time and effort savings
- Increased data quality and integrity
Key Features
- Metadata orientation
- Highly configurable
- Information tracking
- Seamless integration with the Ennov Regulatory Suite
- Robust reporting and analytics
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
