Ennov Solutions
Intrinsically Connected Applications Built on our Unified Compliance Platform
Supporting the Entire Life Sciences Value Chain
Ennov provides Life Sciences and other highly regulated companies with robust content and data management applications that span the entire product life cycle. Built on our unified platform, our solutions are intrinsically connected and do not require system integrations or connectors to facilitate interoperability. A common, harmonized and configurable data model eliminates redundancy and ensures data accuracy and consistency across all applications, while complying with industry best practices.
The Challenges
Integrating disparate information systems can be complex, difficult to maintain, and expensive. Different systems often have different architectures that make integration nearly impossible. Updates to one system can have an adverse effect on other systems, requiring extensive testing and re-validation. Human effort is also needed to share data between these systems: a practice that can be error-prone and introduce compliance risks. Bespoke systems and integrations are expensive, complex, and come with their own sets of issues, most notably system upgrade paths and forward compatibility concerns.
The Solution
With Ennov, integrations to connect disparate and disconnected silos of information become immaterial as all information is inherent to the applications within the solution suite. This approach reduces the overall cost of compliance through the reduction of annual software support and maintenance fees, faster employee ramp-up, and lower re-validation costs.
Come join more than 450 Life Sciences companies around the world being powered by Ennov
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager
World-class Regulatory content and information management
Submission & Artwork Document Management
Manages regulatory submission content based on an industry standard reference model.
Regulatory Information Management
A single authoritative source for substance, product, organization, registration, XEVMPD and IDMP information.
Automate PDF conversion, enrichment and quality control. Accelerate regulatory document preparation.
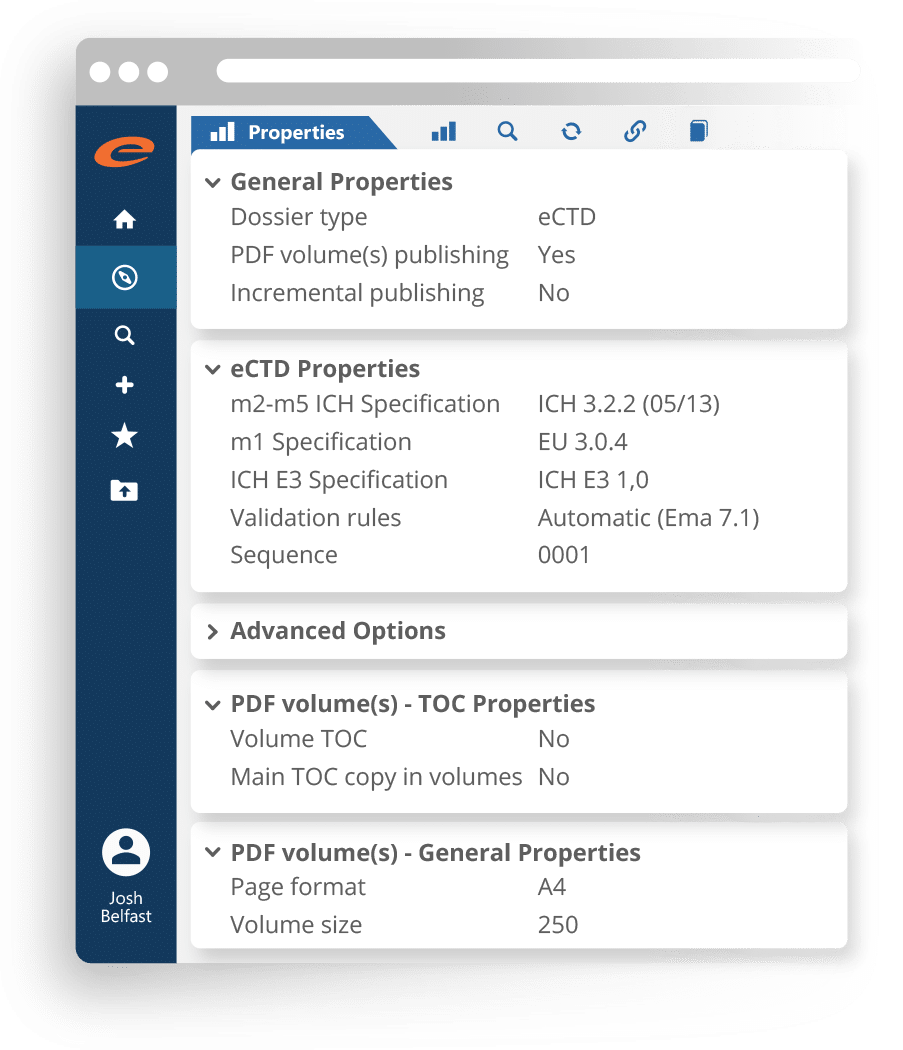
Regulatory Submission Publishing
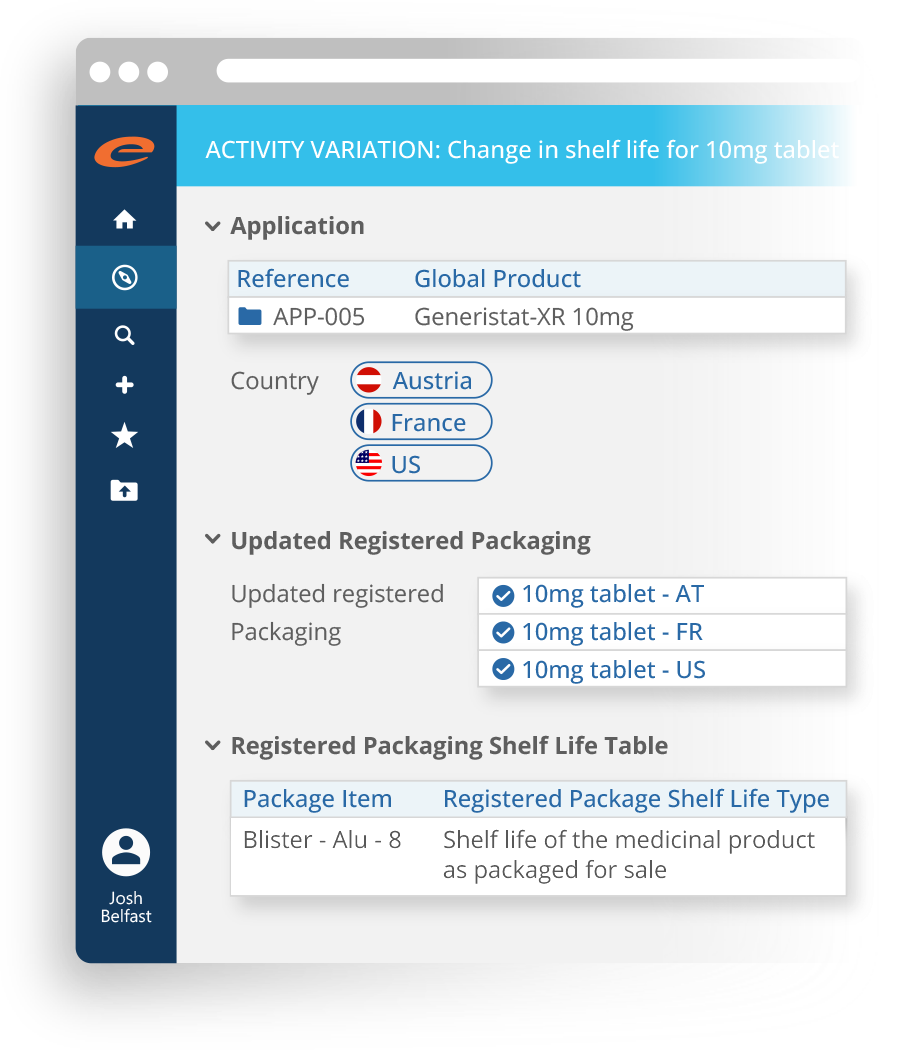
A complete and scalable dossier management and submission publishing solution that is suitable for regulatory operations of all sizes.
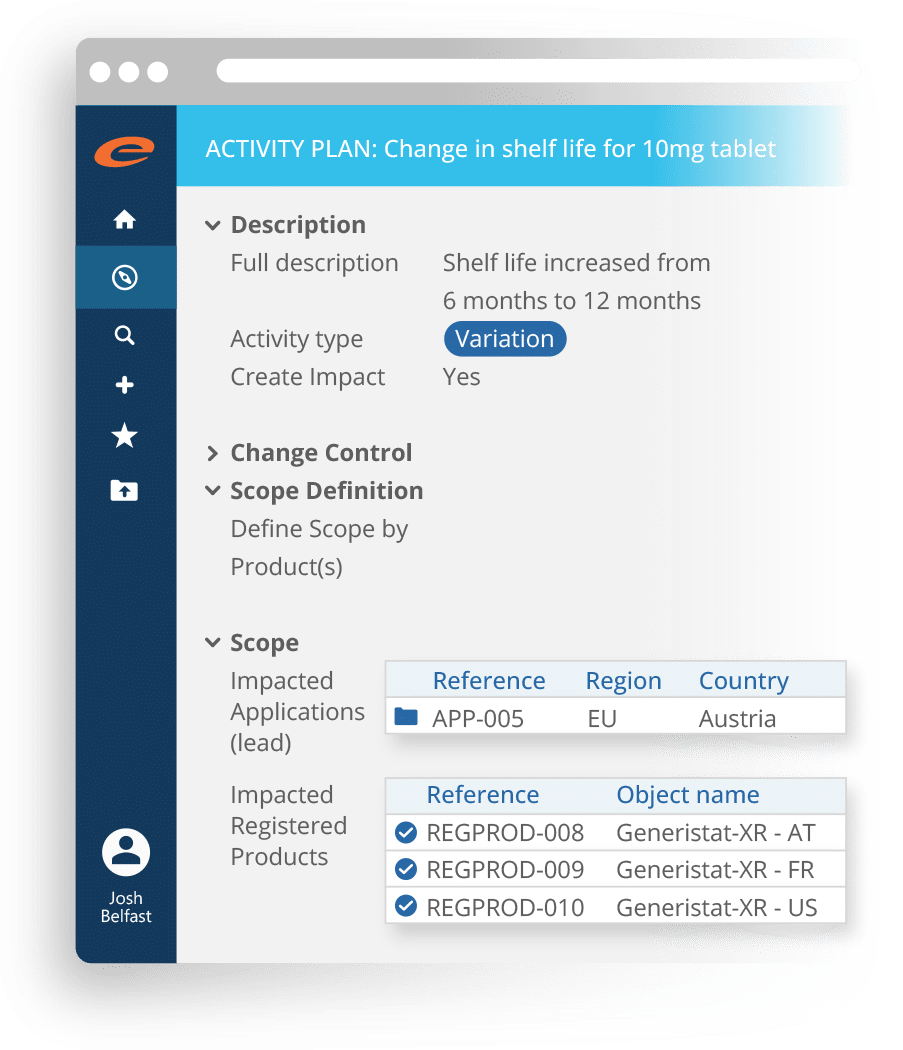
Regulatory Process Management
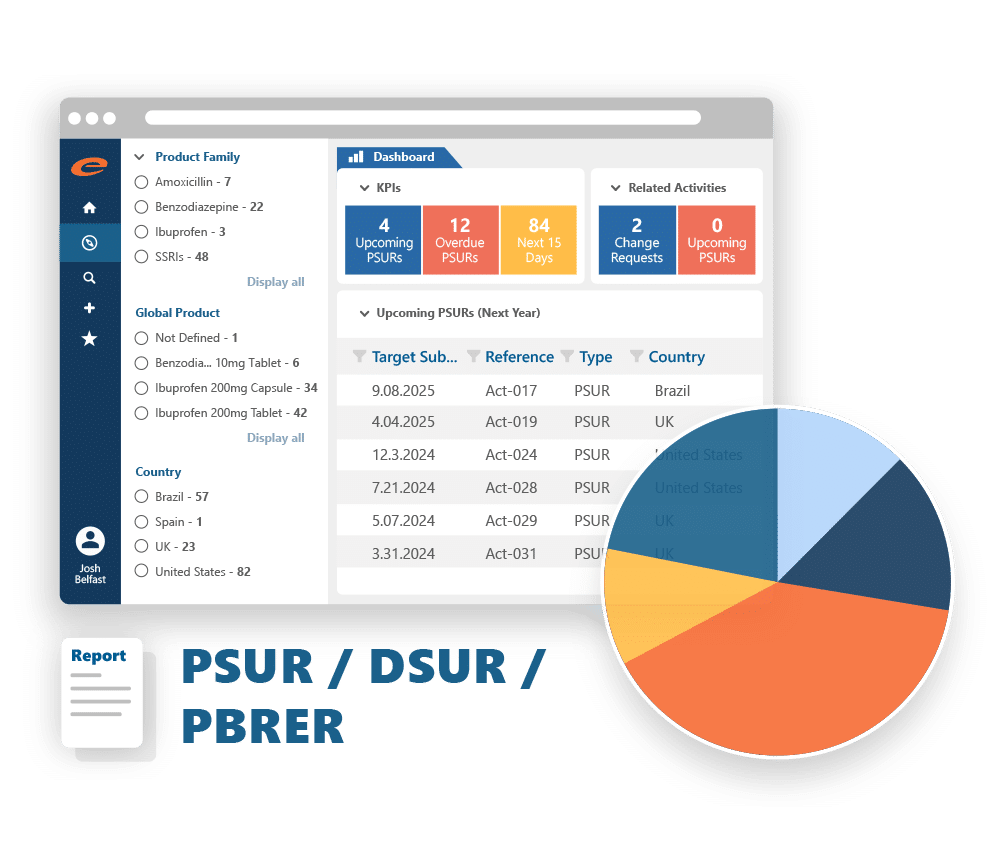
Manages regulatory activities for a product in a market including the original application, variations, supplements, renewals, PSURs, Annual Reports, commitments and correspondence.
Dashboards and Reporting
Understand the global regulatory position of your products, monitor and anticipate regulatory activities and workload, and ensure that key deadlines and deliverables are not missed.
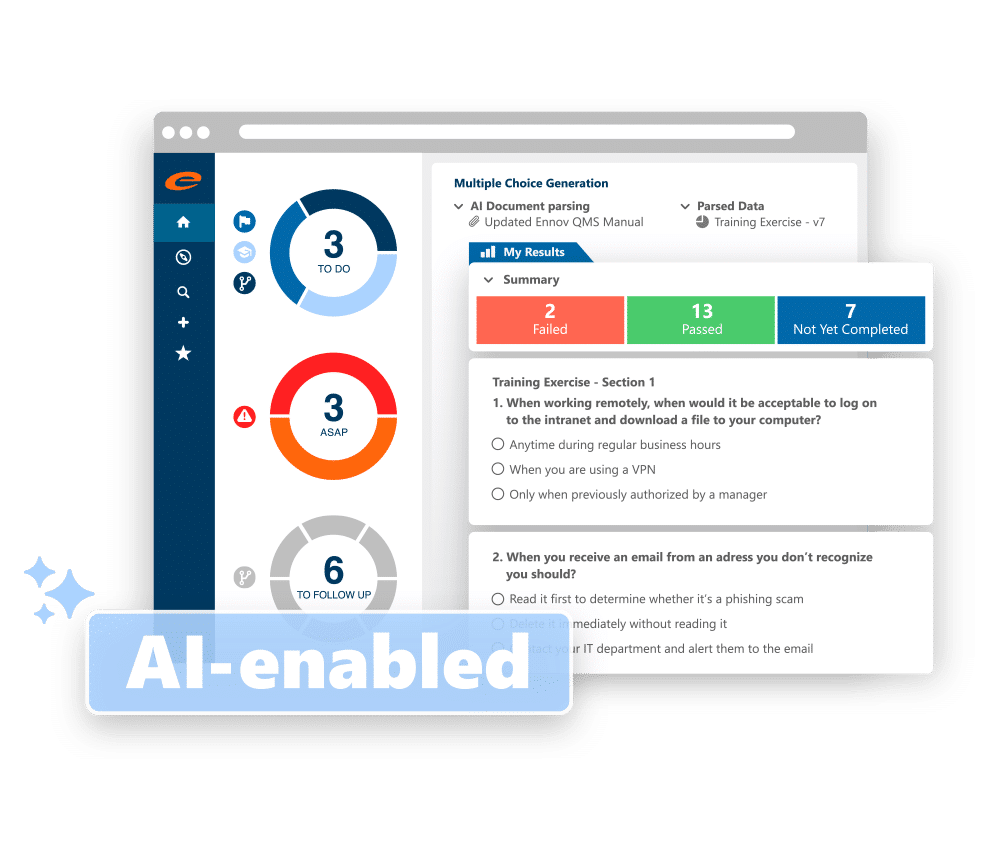
A comprehensive QMS to improve efficiency and ensure compliance
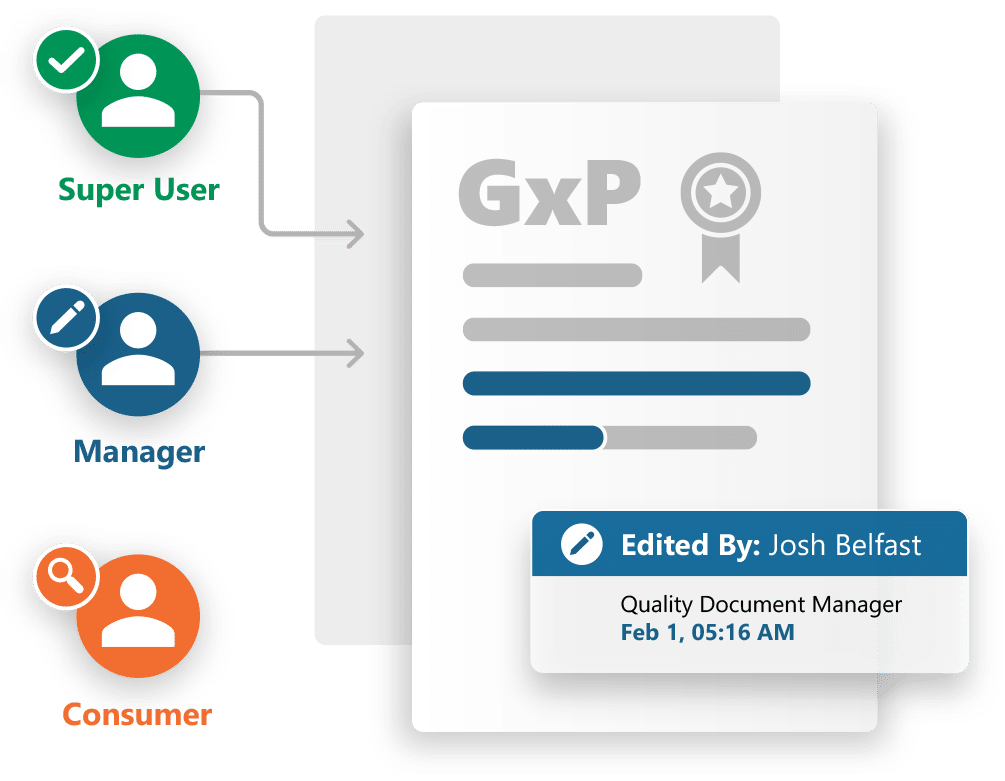
Quality Document Control
Manages regulated GxP documentation based on an industry standard reference model.
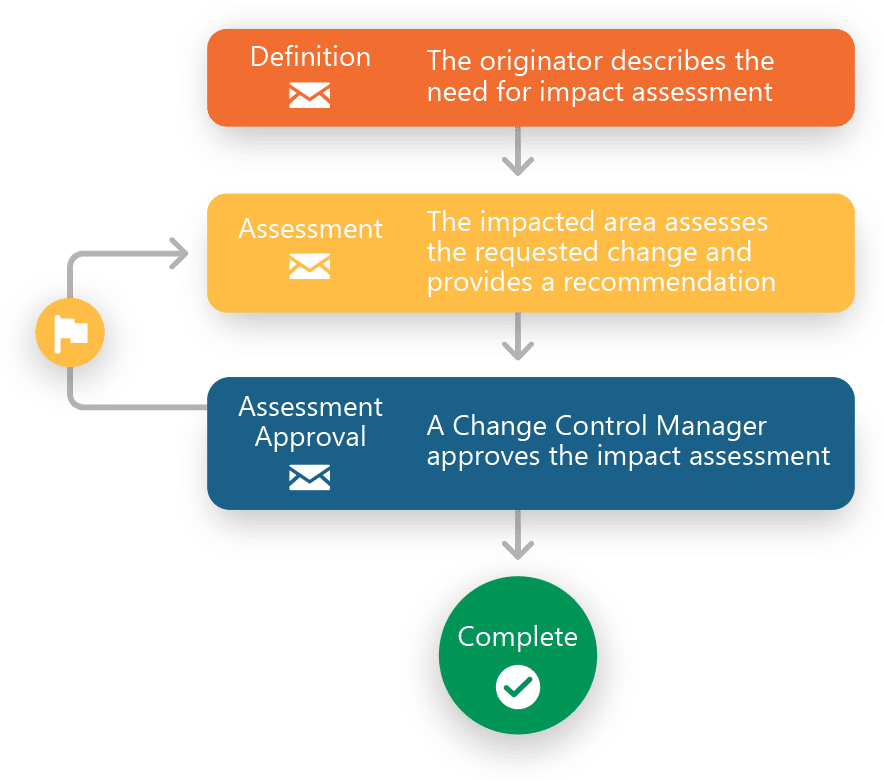
Quality Process Management
Streamlines GxP processes including Change Controls, Audits, Deviations, Complaints, OOS and CAPAs.
Supplier Management
Manages and tracks the performance of suppliers and distributors to maximize performance, reduce risk and manage costs.
Learning Management
Automates the management of GxP training content, learning curricula and AI-generated proficiency assessments to ensure compliance.
Dashboards and Reporting
Gain valuable insights into QA/QC operations to identify and prevent problematic trends before they lead to quality issues.
The professional solution to managing Events, Transparency, Contracts, and Promotional Materials
Events and Transparency
Comply with all relevant laws and guidelines regarding HCP interactions, disclose all HCP related event expenses, and maintain comprehensive records of attendee information, speaker details and event agendas.
Contract Management
Compose and edit templates for contracts, letters of agreement and other event related documents.
Promotional Materials Management
Ensure that all marketing materials comply with stringent regulatory requirements, minimize legal risks and guarantee that healthcare professionals receive accurate and up-to-date information about products.
Dashboards and Reporting
Track key metrics like attendance, engagement levels, post-event surveys, and feedback to measure event effectiveness.
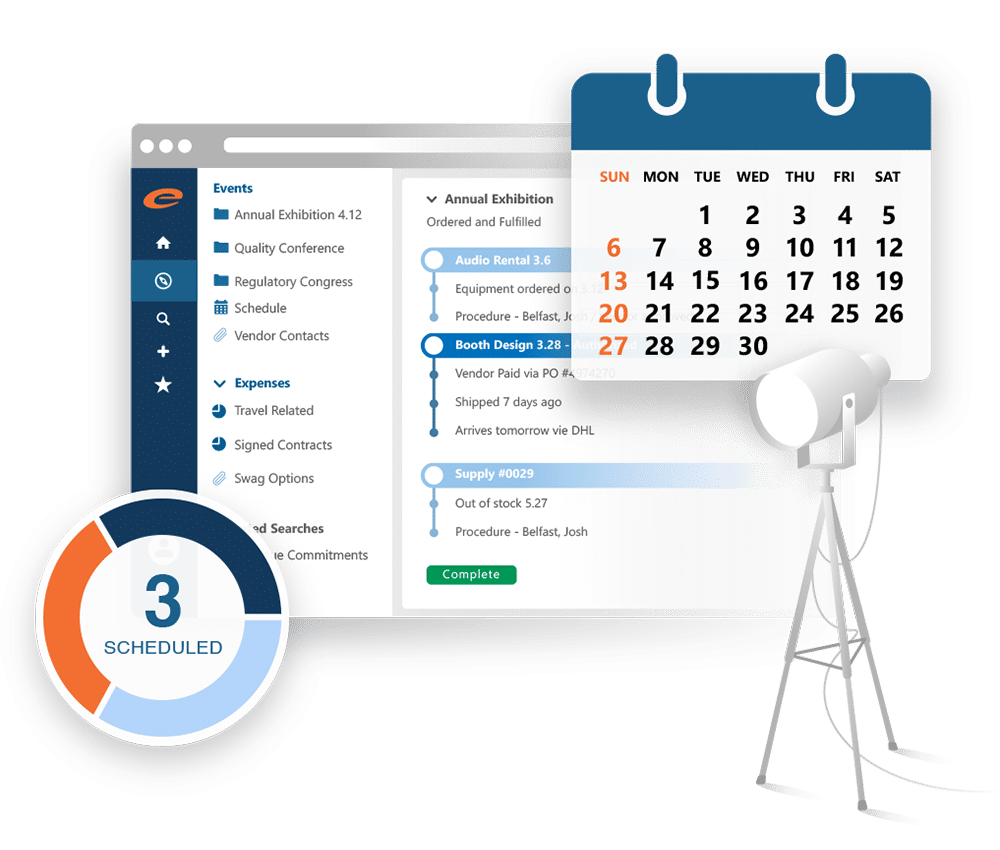
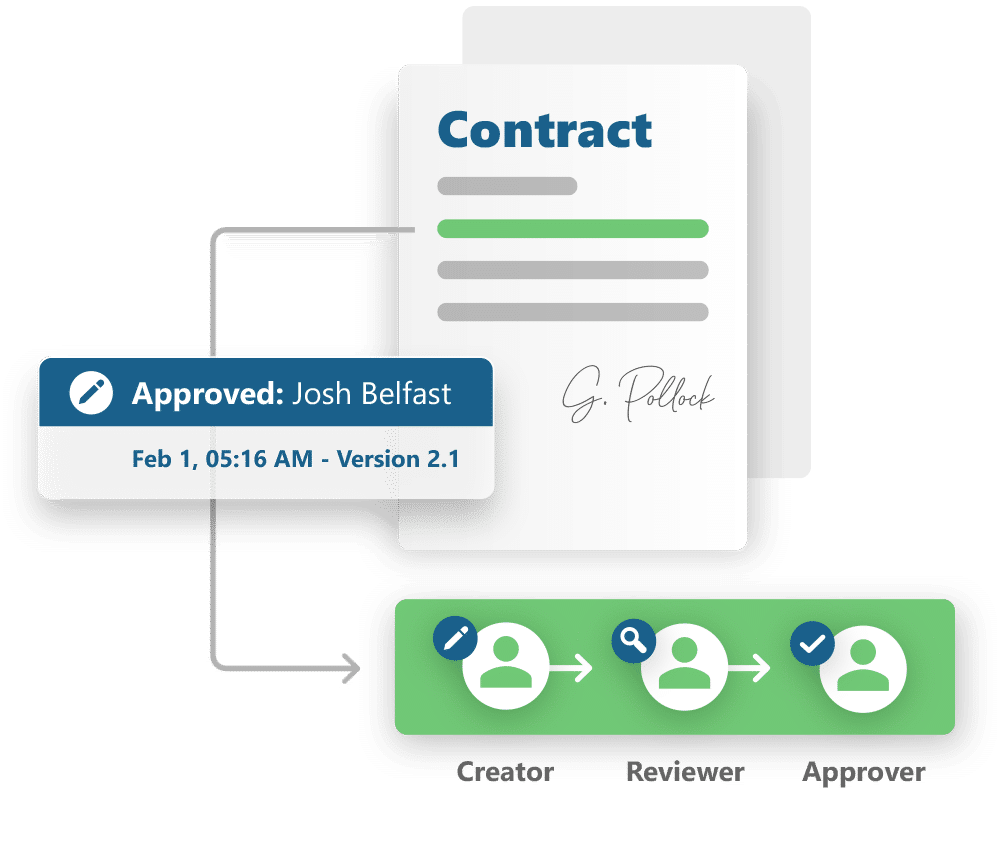
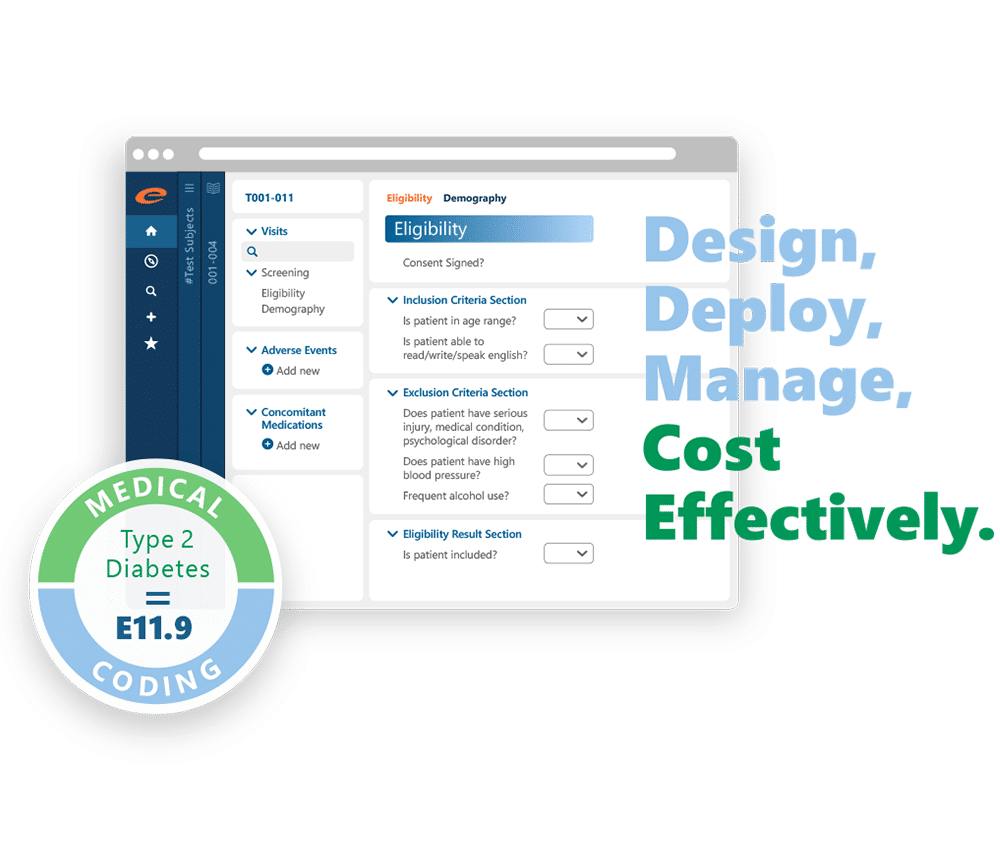
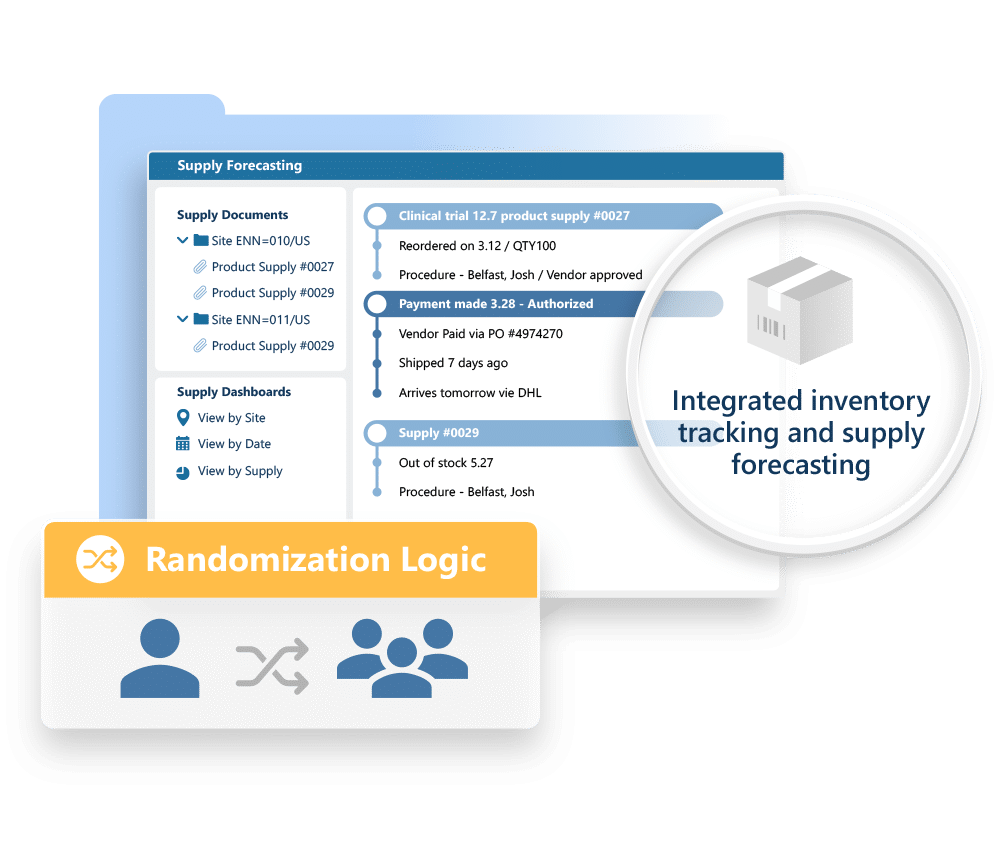
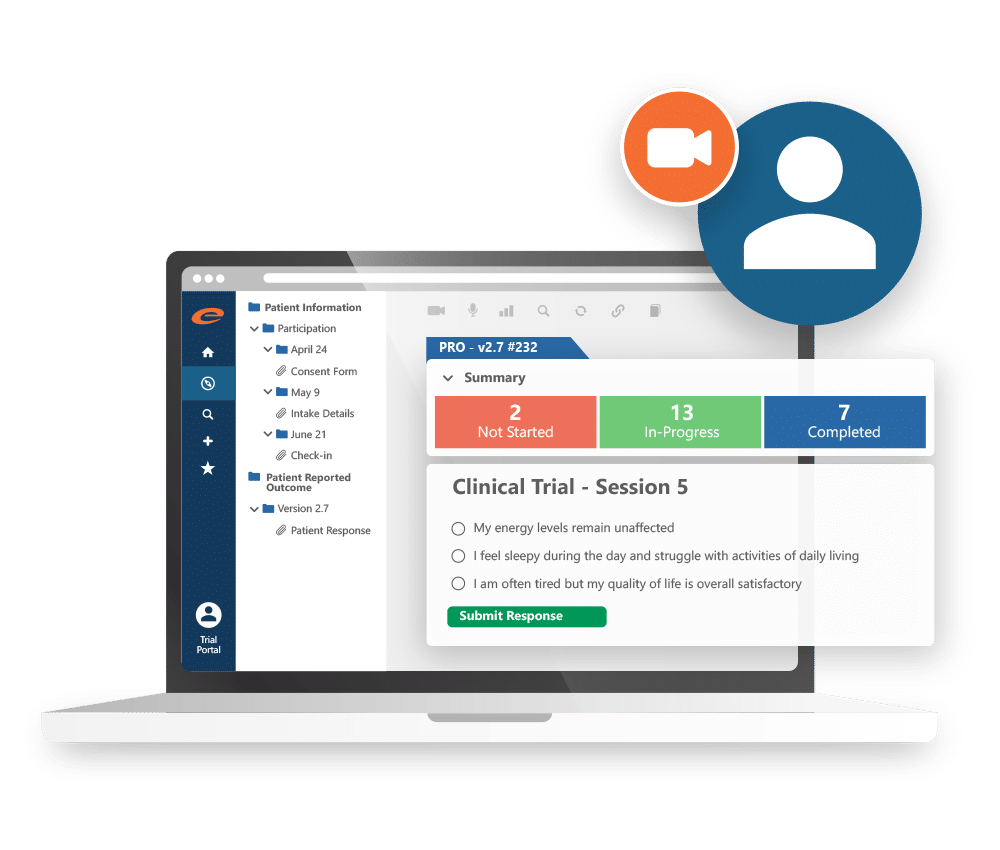
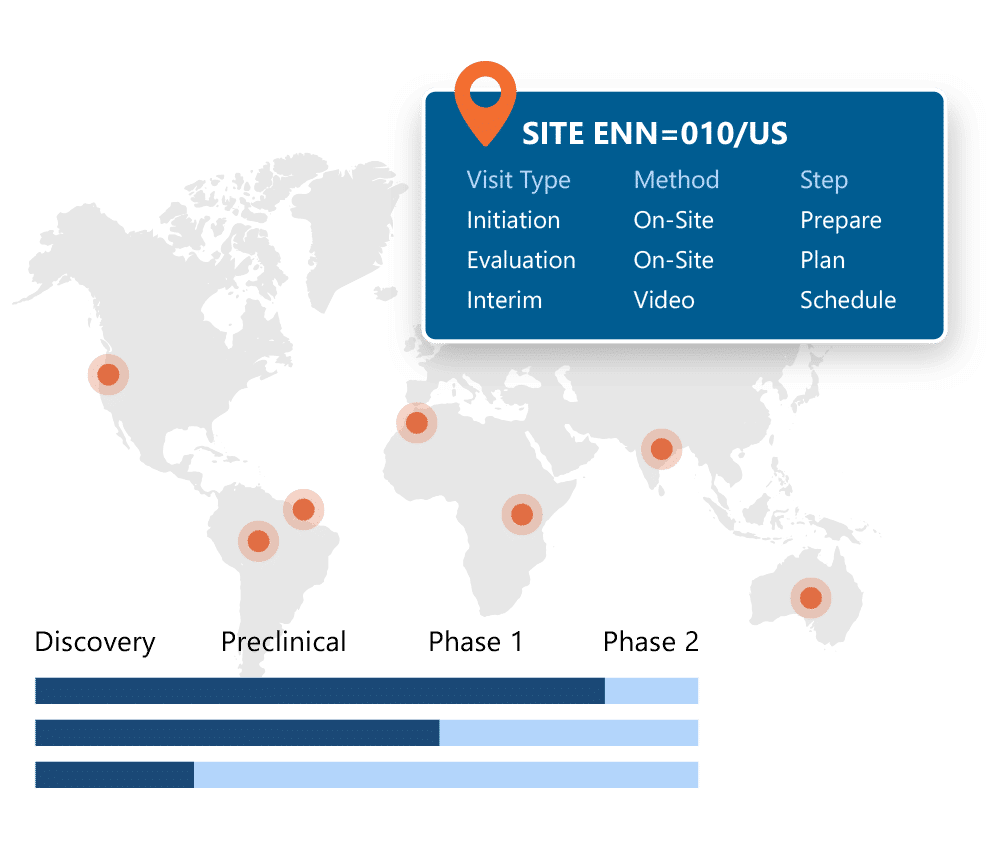
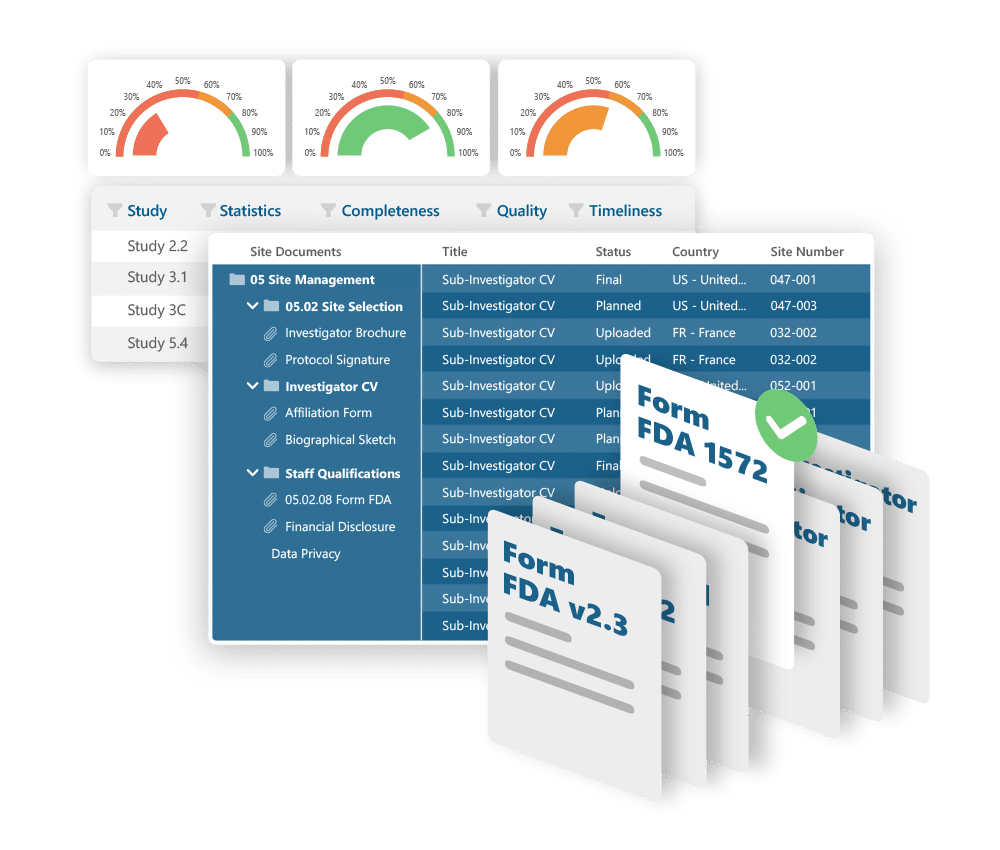
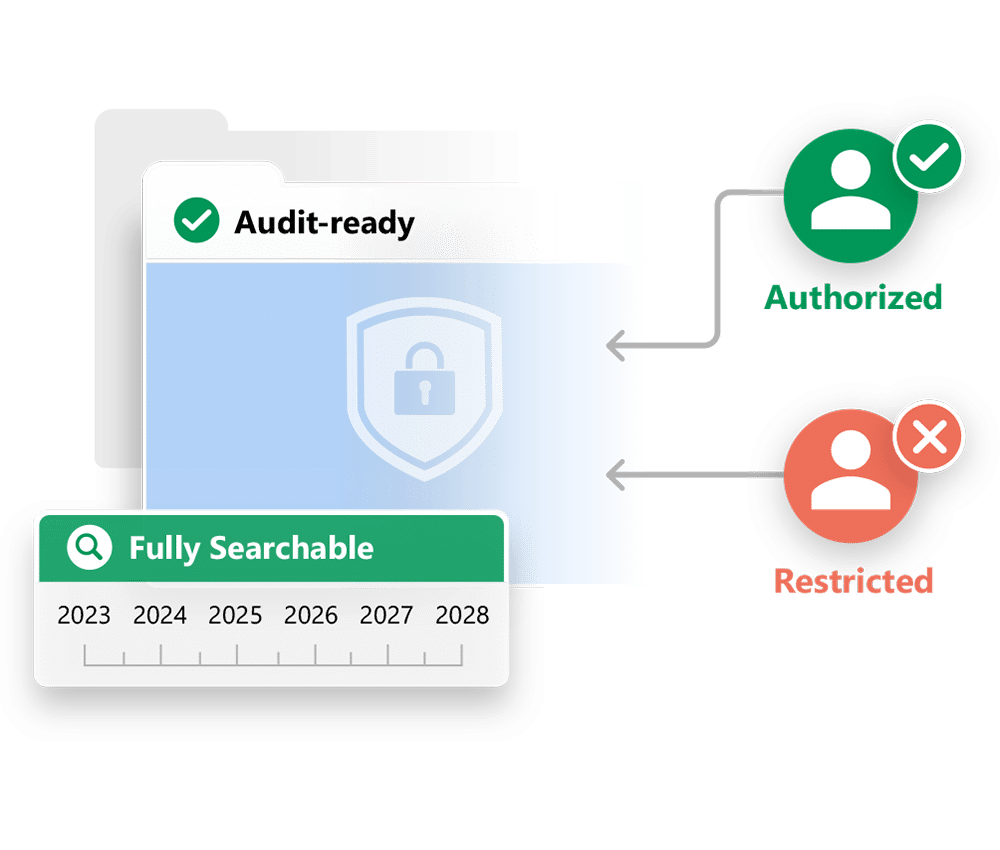
A total solution to capture and manage Clinical Trial information
Clinical Trial Management
Allows trial sponsors and CROs to manage investigative sites to ensure efficiency, quality and adherence to protocols.
Randomization and Trial Supply Management
Streamlines trial operations, improves data integrity, reduces waste, and enhances compliance by automating critical processes.
Electronic Data Capture
Improves data accuracy and consistency, ensures compliance with regulatory requirements, and protects data integrity and security.
Electronic Patient Reported Outcomes
Improves symptom management and quality of life while helping patients avoid serious adverse events.
Electronic Trial Master File
Collects, stores, tracks, and archives documents – demonstrating that the trial was conducted in accordance with regulations and protocols.
Dashboards and Reporting
Early identification of potential problems allows Study Managers to make informed decisions quickly and facilitates better collaboration between the different teams involved in the trial.
End-to-end human and veterinary PV data collection, reporting and analysis
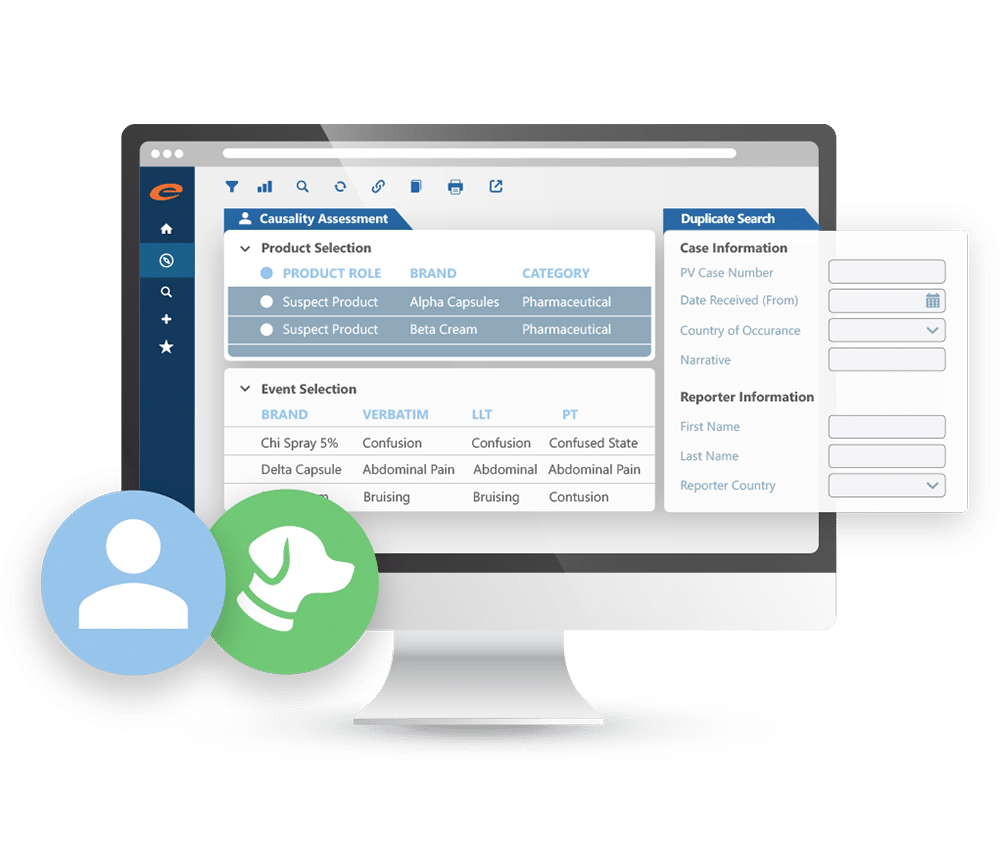
Case Intake and Processing
AI-enabled collection, assessment, and filing of all incoming reports of adverse drug reactions and other safety-related events.
Case Reporting
Efficiently creates and submits Adverse Event case reports in accordance with global Health Authority requirements.
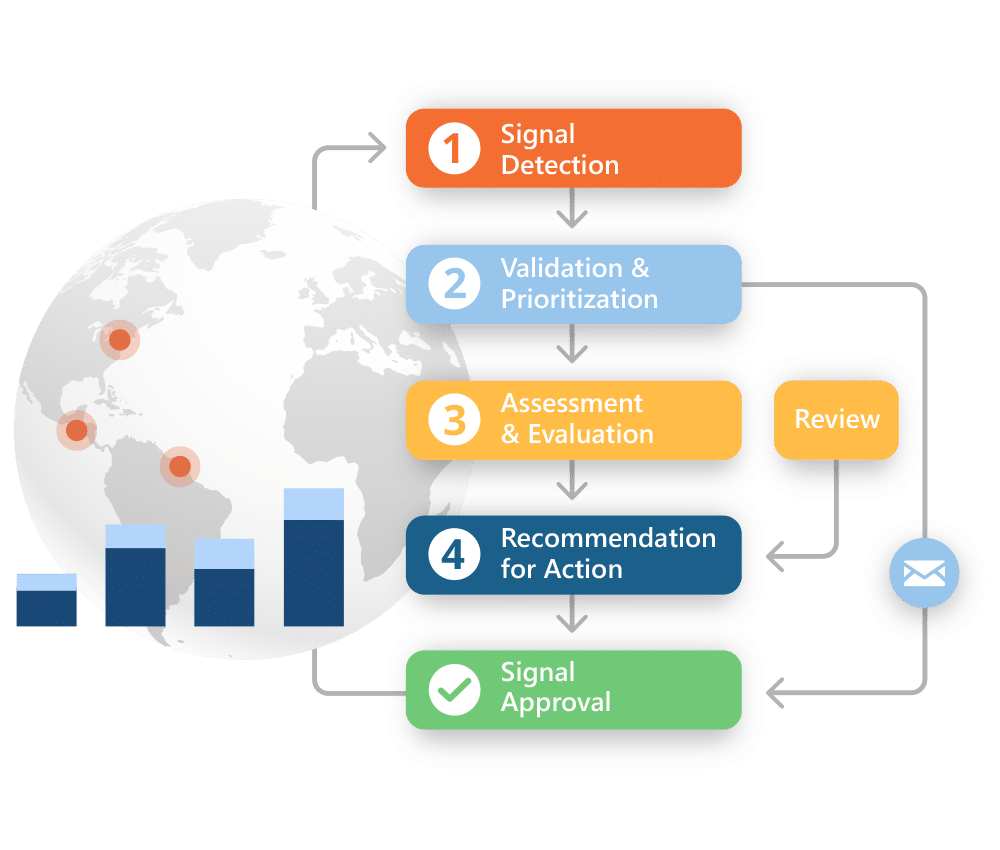
Signal Detection
Analyze data from a variety of sources to identify patterns and associations between the drug and adverse or beneficial events.
Signal Management
Prioritize signals and take action to assess and mitigate potential risks.
Dashboards and Reporting
Present complex data in easily digestible charts and graphs to facilitate rapid interpretation and understanding of safety trends and enhance decision-making.