Clinical Data Management
A Comprehensive Solution to Capture and Manage Clinical Trial Information
- Intuitive, no-code study design and eCRF configuration
- Built-in validations and edit checks to improve data quality
- Seamless integration with Ennov ePRO for direct patient input
- Automated randomization and medical coding (MedDRA, WHO Drug)
- Fast data imports, clean exports, and reliable traceability
- Supports rapid deployment and lower study build cost
Benefits
- One unified platform
Manage clinical data, documents, workflows, and oversight within a single, integrated suite. Simplify operations and eliminate silos across systems and teams. - Fewer manual tasks, greater productivity
Automate repetitive processes—like data validation, site payments, and monitoring visit reports—to free up time and reduce risk of error.
- Designed for teams of any size, anywhere
Whether you’re running one study or dozens, Ennov supports collaboration across regions, teams, and partners—with intuitive tools and multilingual support built in. - Real-time visibility, better decisions
Access dashboards and analytics that provide actionable insights into trial progress, site activity, and compliance risks—before they become costly problems.
The Clinical Data Capture Challenge
Every clinical researcher understands the importance of saving time and resources during the conduct of clinical trials. This is especially true when you consider the collection, processing and management of protocol-specific data for each study subject. In the past, study coordinators relied on paper Case Report Forms (CRFs) to ensure the required patient data was recorded and transferred to the sponsor for processing and analysis.
Thankfully the days of paper CRFs are behind us as the industry understands that the use of electronic data capture (EDC) systems increases the efficiency of data collection and improves overall data quality. In addition to increased speed and quality, EDC also provides faster data access, improved security and greater visibility while reducing costs and ensuring regulatory compliance. All EDC solutions are not created equal so selecting the right one is very important. Your EDC system should be flexible, highly-configurable, scalable, easy to use and, most importantly, compliant with all requisite regulatory requirements and industry standards.
A Complete Solution for Clinical Trial Data Capture and Management
Ennov EDC is a comprehensive clinical data management solution that allows clinical research personnel to easily define EDC studies and collect subject data without the worry of missing or inaccurate data.
Ennov EDC is easy to use and supports the design, deployment and capture of multi-center clinical trial data within one flexible and scalable solution. Our software supports clinical trials of any size, including large global trials, post-marketing trials, cohort trials, health surveillance, Phase I-IV trials and epidemiological trials. Ennov EDC facilitates complete and accurate data collection and improves communication within clinical teams
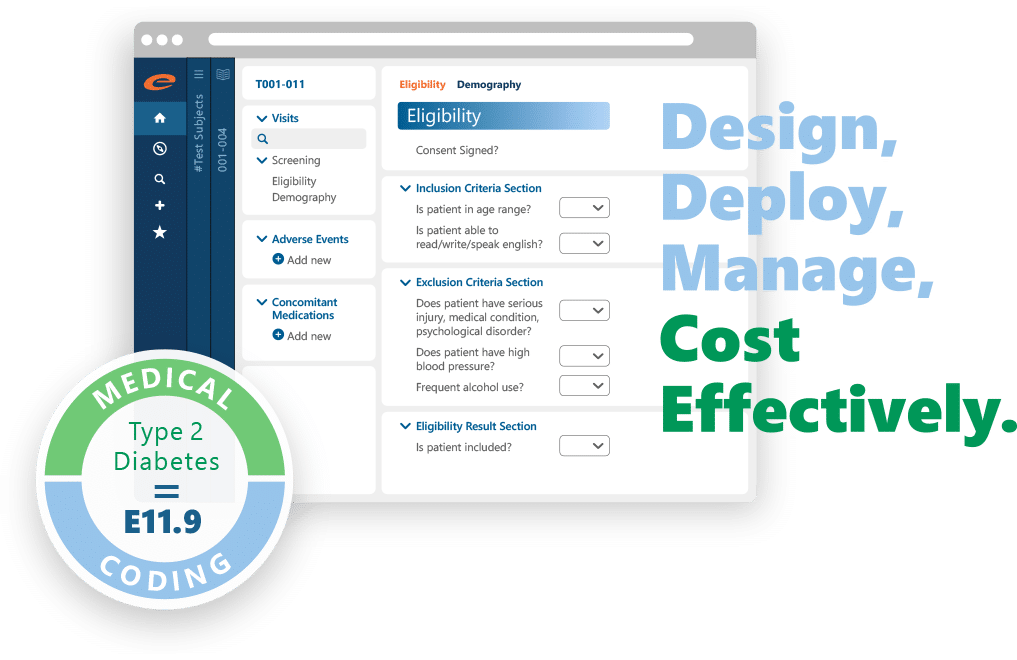
A Complete Solution for Clinical Trial Data Capture and Management
Ennov EDC is a comprehensive clinical data management solution that allows clinical research personnel to easily define EDC studies and collect subject data without the worry of missing or inaccurate data.
Ennov EDC is easy to use and supports the design, deployment and capture of multi-center clinical trial data within one flexible and scalable solution. Our software supports clinical trials of any size, including large global trials, post-marketing trials, cohort trials, health surveillance, Phase I-IV trials and epidemiological trials. Ennov EDC facilitates complete and accurate data collection and improves communication within clinical teams
Accessible for any device.
Ennov EDC is designed for clinical investigators and site staff, enabling seamless data entry and navigation from any device, desktop, tablet, or mobile.
Creating patient visits, completing eCRFs, and reviewing patient files are simple and intuitive, with a responsive interface optimized for all screen sizes. Users can also capture and upload images as source documents directly into the eCRF when using devices with camera functionality.
Why Ennov is Better
Data must be shared between different departments, activities and systems. This data can be considered as a central asset of the company, whose control, or lack of control, has direct consequences on its ability to operate effectively in its market. However, as we have also seen, the interactions between data obey extremely complex models.
As a result, Master Data Management projects are essential for many companies in the pharmaceutical sector. The aim is to set up data management systems to ensure that data is consistent and relevant to all the uses to which it can be put within the company. MDM solutions define, data by data, which system is the master, which system is the slave, and how the data are linked together.
Ennov will be able to rely on an MDM when it is in place, in order to provide or consume data. When there is no MDM, Ennov’s RIM can perform this function.
Core Capabilities
- Quick and easy eCRF design
- Support for all data field formats
- Computed data fields and interval calculations
- Defined data field groups
- Dynamic data field activation
- Support for optional eCRF pages
- Configurable library of allowable values
- Online or offline patient data entry
- Attach photos to eCRF using iPad camera
Key Features
- Streamlined clinical data capture
- Requires no IT or programming skills
- Increased data visibility and security
- Medical coding for MedDRA and WHO Drug
- CDISC, CDASH and SDTM compliant
- Full web interface
- 21 CFR Part 11 compliant
- Available in the App Store
- Compatible with existing Ennov Clinical studies
The Randomization and Trial Supply Management Challenge
Randomization is a fundamental requirement of clinical trials. In recent years the demand for more randomized clinical trials has increased in many areas of clinical research. Randomization is important for several reasons but most importantly it ensures treatment group balance, eliminates selection bias and limits the predictability of treatment allocation.
Another critical requirement is trial supply management. The planning, manufacturing and distribution of clinical supplies can be complex due to a number of factors. However, at the end of the day, clinical trial supply management must ensure that the right medical supplies are delivered to the right patient at the right time, every time.
Ennov RTSM: Two Challenges – One Solution
Ennov RTSM is a comprehensive solution that manages randomization and clinical trial supplies. An integrated IWRS (Interactive Web Response System) allows clinical investigators and site personnel to access study data from any location at any time and execute their study-specific activities using an intuitive web-based interface.
Ennov RTSM allow statisticians to define complex randomization schemes and supports patient randomization using standard algorithms such as minimization and stratification (variable lists). Ennov RTSM supports virtually all study types including blinded studies, double blinded studies, multi-arm, and multi-arm multi-stage studies. Patient randomization by the clinical investigator is performed quickly and easily by connecting to Ennov EDC via a web browser.
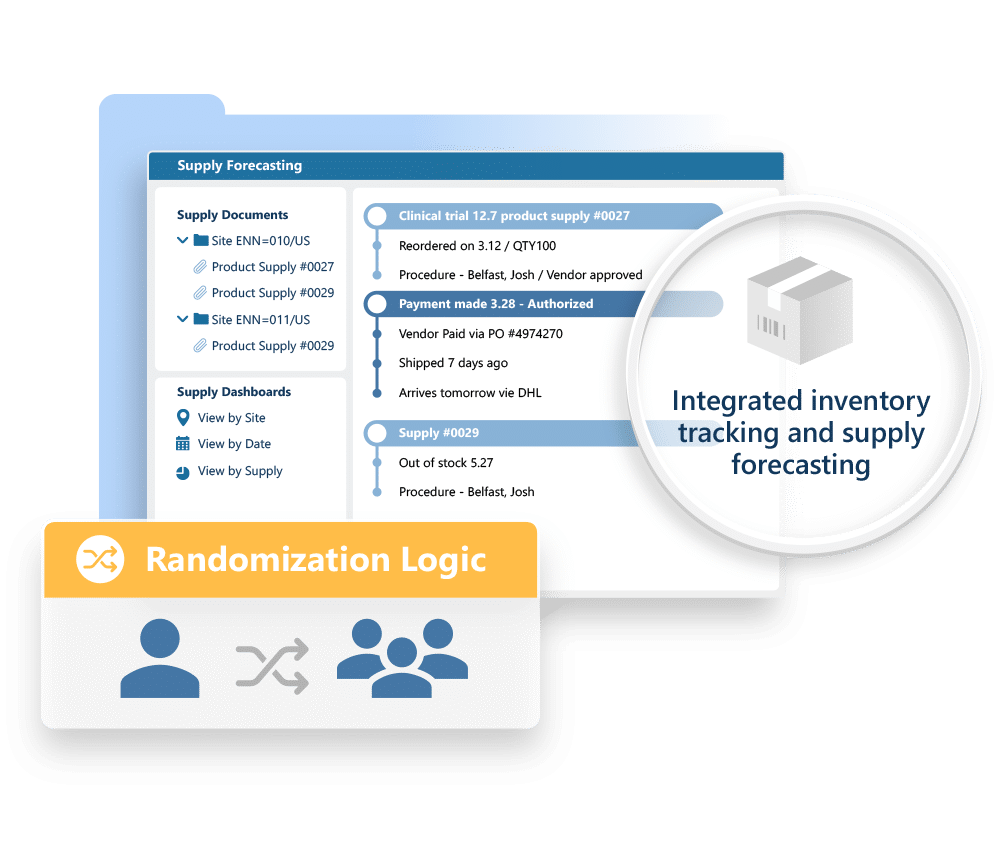
Ennov RTSM is also used to manage the IMP (investigational medicinal product) from the initial shipment to the investigating centers to dispensing and replenishment. E-mail alerts on the IMP stock status provide site coordinators with complete transparency into their on-site inventories and automatic replenishment keeps their studies running smoothly and without disruption.
Flexible and Comprehensive
Randomization and capturing clinical data go hand-in-hand, which is why the Ennov RTSM connects directly to Ennov EDC. When conducting an EDC study, the investigator can allocate the treatment (either placebo or IMP) to a patient according to a randomization list or via a minimization algorithm. Using a web browser, The investigator connects to Ennov EDC to add a patient to the clinical trial. After entering the patient eligibility criteria and other parameters required for stratification, the investigator requests the randomization for the patient.
Data managers can easily define the randomization scenarios with Ennov RTSM. Many configurable options are available including the choice of which page the investigator will request randomization, blindness management, choice of strata, type of randomization, option “random factor”, and option “X random patients”.
Ennov RTSM allows you to directly display the result of randomization in the eCRF such as the treatment arm or any references to the experimental drugs that need to be given to a patient.
Core Capabilities
- Randomization control with consistency checks and email alerts
- Complete traceability of sudies, patients and status
- Integrated IWRS functionality
- Flexible randomization scenarios
- Support for any study type
- Clinical supply management
- Treatment allocation
- Blind management
Key Features
- Unlimited number of strata and treatment options
- Display randomization results in eCRF
- User friendly and easy to use
- Integrated with Ennov EDC
- Global follow-up with email alerts
- Flexible and configurable
- 100% web-based interface
- 21 CFR Part 11 compliant
The Patient Data Collection Challenge
Paper-based clinical trials are inefficient, expensive and error-prone. This is particularly the case when using paper diaries and questionnaires to collect patient data and access clinical outcomes. Paper diaries are cumbersome to use and can lead to data integrity issues. Many times the paper forms are filled out incorrectly or incompletely, leading to unnecessary delays while the the data excursions are researched and corrected. Transcription from paper-forms into data management systems is not without it’s own set of risks and complicating factors.
The cumbersome nature of paper makes patient compliance difficult to enforce. Recent studies have shown that 90% of clinical trial patients prefer to use an electronic platform to record their information. In fact, patient compliance levels of over 90% are common with electronic trials while compliance levels for paper-based trials are in the 10-15% range.
Ennov ePRO: A Digital Alternative to Paper Diaries
Ennov ePRO facilitates the direct capture of electronic patient data without the worry of access, recovery or security. Patients enter their data easily using an intuitive, user friendly web application. The data is immediately checked for validity, consistency and completeness and is then made available to the investigators and site personnel responsible for monitoring patient compliance and safety.
Patient self assessments and collecting quality of life information is made easier with Ennov ePRO. Navigation within questionnaires is easy and intuitive. Patients can easily understand the interface and access the system from the comfort of their home. Our Visual Analog Scale (VAS) allows patients to effectively report pain levels or other information that is typically collected in a graduated manner. Throughout a study, patients are automatically notified (via personal messaging) of the availability of new questionnaires to complete—accelerating the trial while improving data quality.
Ennov ePro can also be used in veterinary studies where behavior and feeding information can be entered by the pet owners.
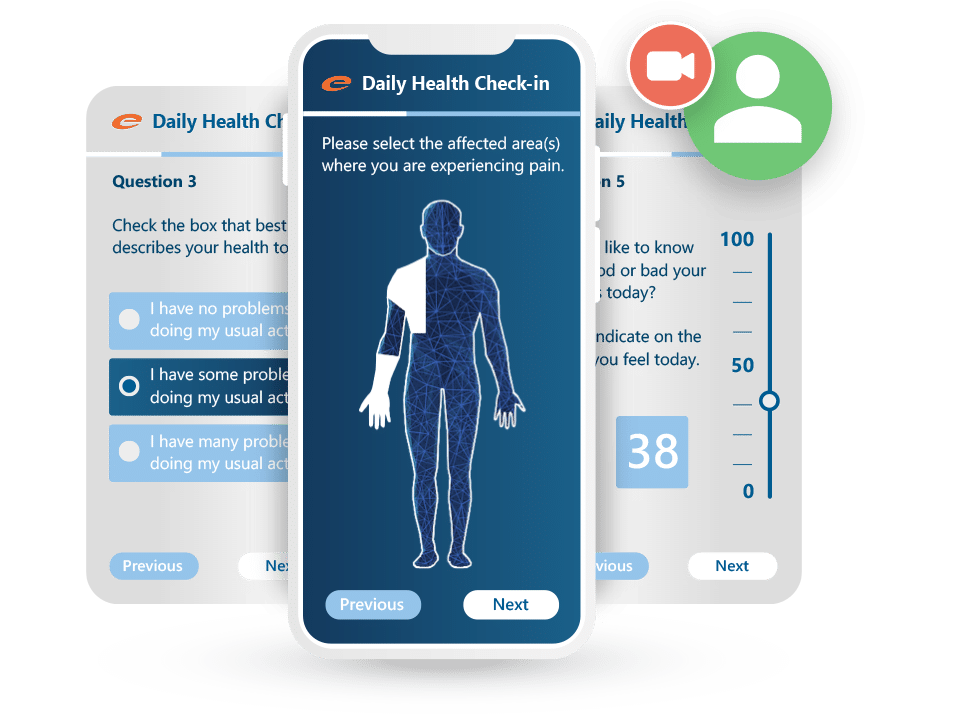
Safe, Secure and Compliant ePRO software
Ennov ePRO ensures that patient data is secure and patient privacy and confidentiality is protected. All ePRO data is encrypted and stored separately from medical data. Ennov ePRO is compliant with all FDA and EMEA regulatory requirements including 21 CFR Part 11.
Core Capabilities
- Intuitive and easy to use interface
- Configurable questionnaires
- Visual Analog Scale for self assessments
- Secure and encrypted
- Real time access to patient data
- Automated notifications
Key Features
- Fully integrated with Ennov EDC
- Saves time and money
- Higher patient compliance levels
- Increased data quality
- 100% web-based
- 21 CFR Part 11 compliant
Efficiently & Securely Capture & Manage Clinical Trial Information
The Ennov Clinical suite consists of Clinical Data Management applications as well as Clinical Trial Management applications that are available for deployment in the cloud or on premises.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
