Ennov PV
Signal Detection and Management
Spot the signal. See the trend. Stay ahead.
- Detailed analyses and graphical output with key statistical measures
- Fully searchable repository of all signal management activity
- Dashboard provides signals in various stage of work-up “at a glance”
- Review data and evaluate signal evolution
- Highly configurable workflows that align with your risk management processes
- Integration with qualitative and quantitative review of adverse event data
Signal Detection
The Signal Identification Challenge & the Ennov solution
Implementing a process to identify potential signals is essential. Ennov’s PV solution contains a comprehensive signal detection and data mining tool. The application, designed for use out-of-the-box by business users, offers a broad array of statistics as well as powerful data cubing and visualization functionality.
With the solution you can rapidly calculate key statistical measures that are widely used by regulatory authorities such as PRR, ROR, and MGPS and apply smart stratification to examine the database and detect where customizable thresholds have been exceeded. The application integrates easily with other PV solutions, and various external data sources to increase flexibility in preparing such important metrics.
Core Capabilities
- Pre-configured document inventory
- Advanced life cycle management
- Flexible rights management
- Automatic PDF rendering
- Scanner integration
- Pre-configured views and tracking dashboards
- Required documents list
Key Features
- Integrated worklist dashboard
- Configurable document types, workflows and views
- Automated email notifications
- Intuitive user interface
- Integated PDF viewer
- 100% web-based
- 21 CFR Part 11 compliant
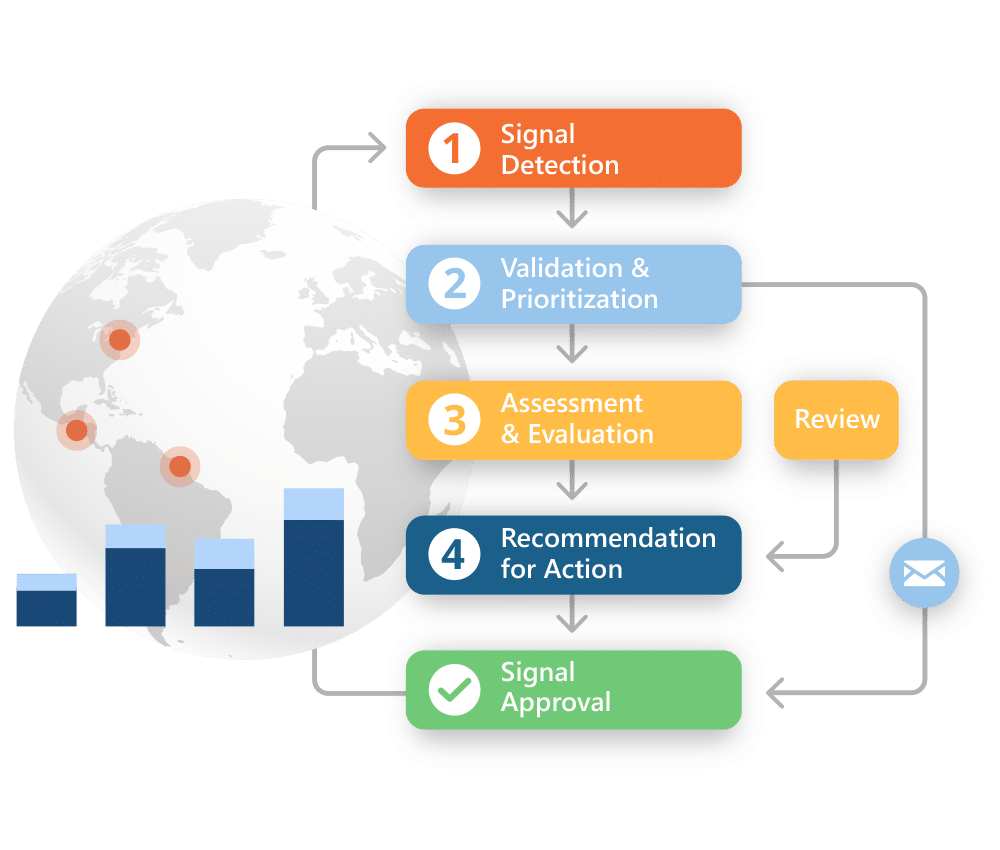
Signal Management
Pharmacovigilance Signal Management Software
Take advantage of a fully searchable repository of all signal management activity, allowing users to review previous data and conclusions and evaluate the evolution of a given signal over time. It includes configurable workflows that are aligned with your risk management processes.
Upon completion of a signal workup, a “Signal Summary Report” is automatically generated, documenting when and how the signal was identified as well as the information collected throughout the signal evaluation and analysis process.
It can be integrated with other innovative Ennov products used for the qualitative and quantitative review of adverse event data. Signals derived from the robust set of built-in statistical analyses included with the solution are populated via our REST API and used to record signal information and its defined risk level.
Core Capabilities
- Pre-configured document inventory
- Advanced life cycle management
- Flexible rights management
- Automatic PDF rendering
- Scanner integration
- Pre-configured views and tracking dashboards
- Required documents list
Key Features
- Integrated worklist dashboard
- Configurable document types, workflows and views
- Automated email notifications
- Intuitive user interface
- Integated PDF viewer
- 100% web-based
- 21 CFR Part 11 compliant
An End-To-End Solution for Collecting, Reporting and Analyzing Human and Vet PV Data
The Ennov Pharmacovigilance solution keep the collection, management, assessment, and reporting of human or veterinary adverse events in one unified database while also providing advanced signal detection and PV data analysis tools.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
