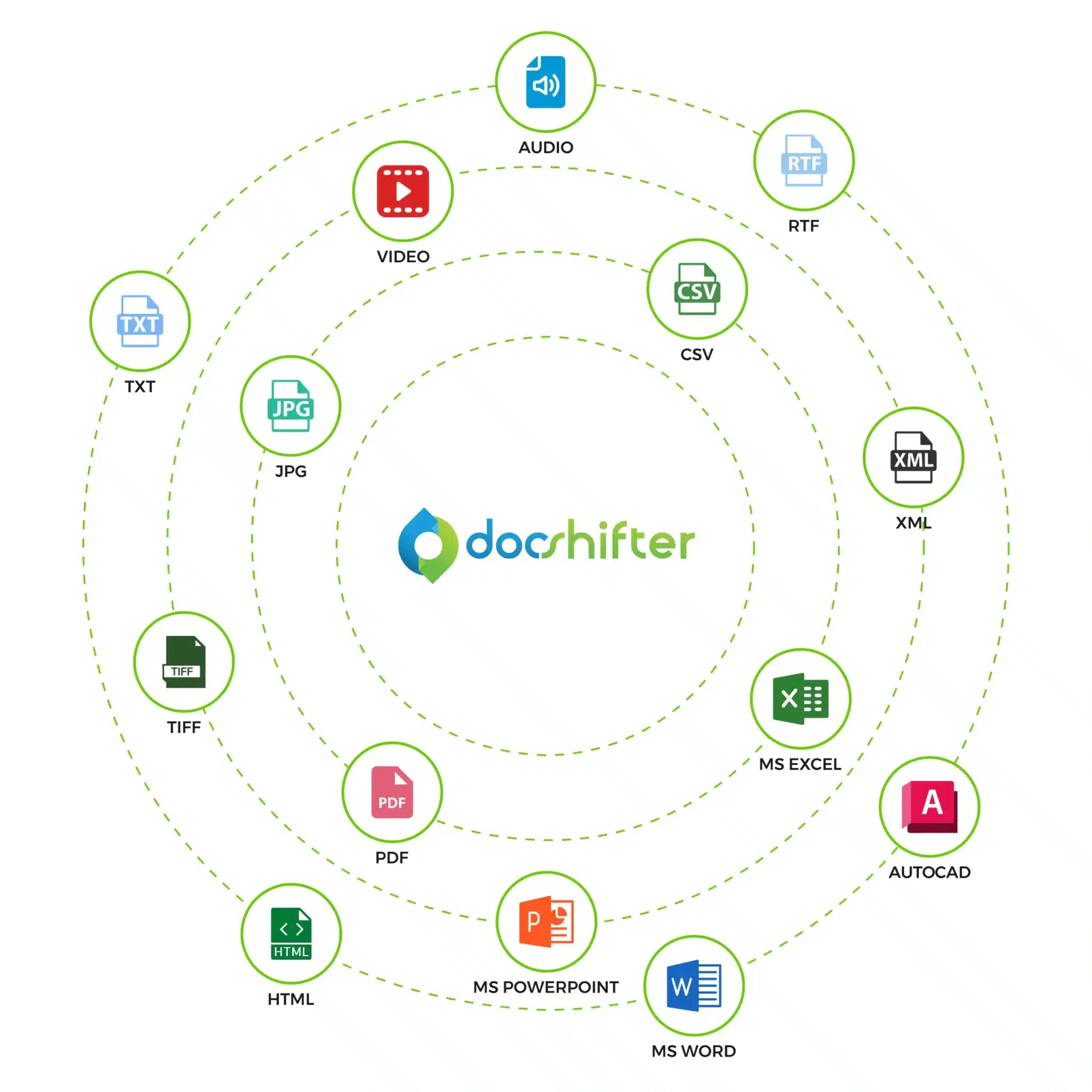
DocShifter:
Market-leading document conversion
Accelerate regulatory document preparation.
Automate PDF conversion, enrichment and quality control.
- Scalable, compliant and fast document preparation
- On-premise or in your cloud
- Integrates seamlessly with all your existing systems
- Trusted by life sciences companies of all sizes
- One solution for all your regulatory and other content transformation needs.
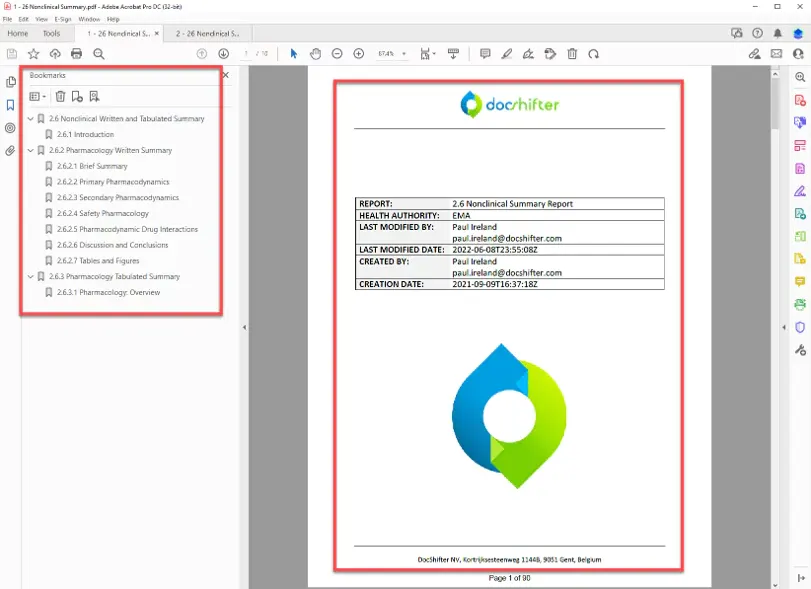
DocShifter automates the entire process of preparing compliant PDFs, including conversion, enrichment, and quality control. The result is faster, more reliable workflows that reduce manual effort and minimize risk.
Whether you need to automate quality check and control of Microsoft Word & PDF files, generate health authority compliant submission-ready PDFs, merge documents into compliant PDFs, generate archive-ready formats, DocShifter automates it all.
The platform handles high volumes with ease and integrates with your existing tools and repositories without disruption.
Whether deployed on-premise or in your cloud, DocShifter is built for speed, scalability, and compliance.
Benefits
- Fully automated
Convert any file type without the need for manual intervention. Simply set up and start converting.
Securely install anywhere
Because security and privacy matter, convert your documents on-premise or in your cloud. On Windows or Docker.
- Superior Conversion Speed
Without the need for MS Office or Adobe, DocShifter converts documents 10x faster than comparable solutions.
- High-availability
With zero downtime, your conversion service will always be on to meet the demands of your business.
Submission-ready PDFs for multiple health authorities
Create what the regulators demand: fully searchable, eCTD compliant PDF renditions, without any manual work
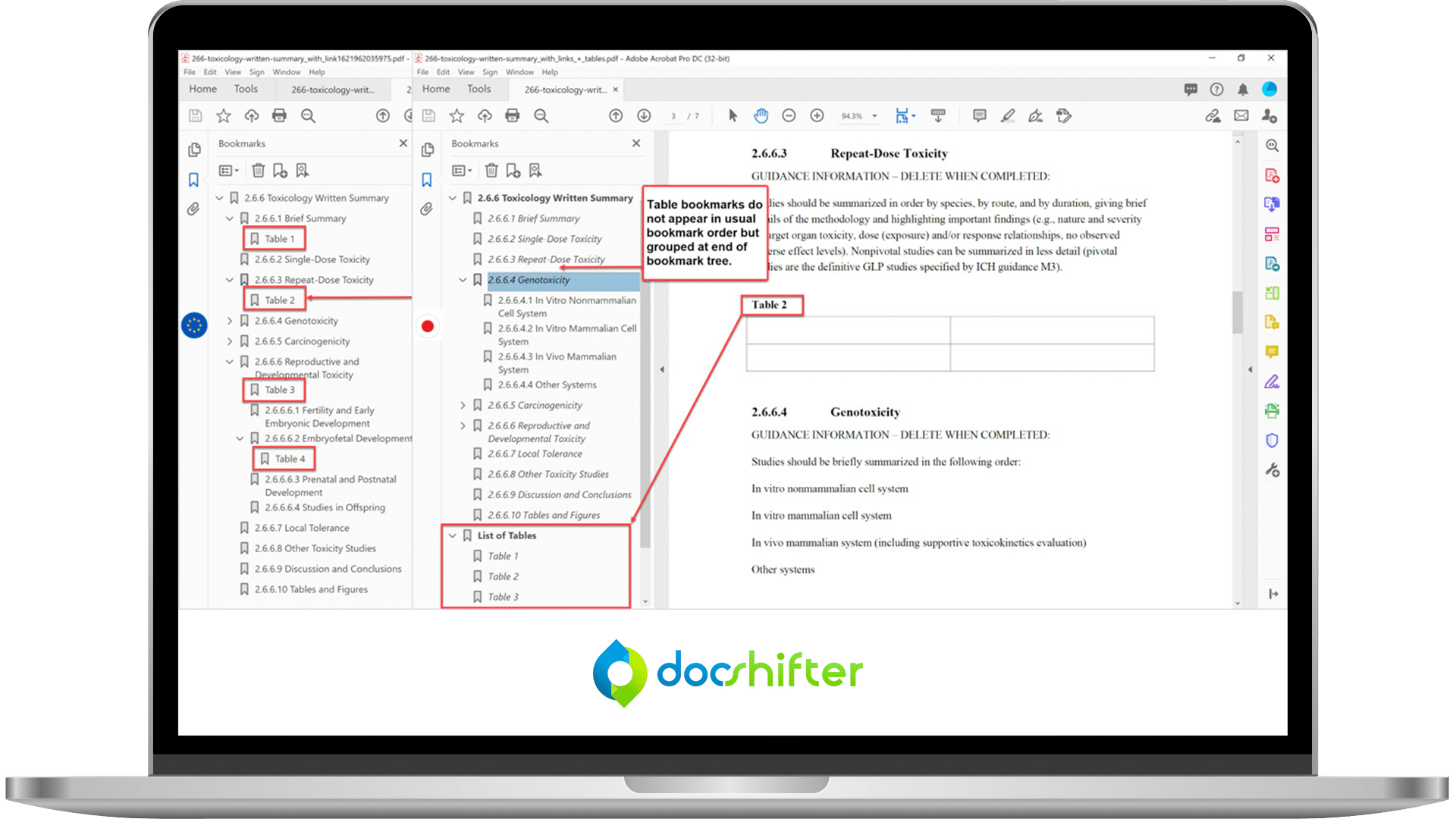
- Advanced bookmark & hyperlink handling
- Detailed PDF specification handling
- Font handling
- Advanced Excel to PDF conversion handling
- Advanced PDF Branding Features
- Flexible Office & Image Handling
Automated report compilation for truly searchable PDF reports
Automatically merge documents into compliant PDFs from your DMS & RIM platform
- Standardized, high quality (compliant) PDF reports
- Connect with your existing system; trigger automation based on lifecycle, metadata, etc.
- Deliver quality-assured, (compliant) reports faster and save time in content preparation
- Add a cover page, tables, watermarks, pagination, headers & footers, and just about everything else you need
- Split reports into multiple output files (based on page count, or merged file size)
Fully automated PDF quality control software
Save time and reduce risk with automated compliance checks for PDF files. Easily check if your PDF is compliant or not.
- Check for bookmark issues
- Check for hyperlinks issues
- Check for PDF properties & security issues
- Check for font and initial view issues
- Check for page related issues
- Check for annotation and text issues
Check & fix Word documents for compliance.
Check Microsoft Word documents for compliance, fix Word formatting and secure submission-ready documents. On time and to the strictest of guidelines.
- Check documents against internal and external guidelines
- Reduce risks and ensure document compliance
- Save time and resource costs with automation
- Produce controlled and consistent results
- Focus more on content creation and less on document formatting
Fully Automated Email to PDF Converter
Monitor Email Inboxes & Streamline Email to PDF Conversion
- Seamlessly Convert Thousands of Emails Daily
- Highly configurable to the specific compliance needs of different industries (life sciences, banking & insurance, etc.)
- Generate compliant PDFs consistently and tackle regulatory affairs head on. Convert to both PDF and PDF/a
- Fully automated email to PDF conversion. Convert in hours not days
- Enable long-term preservation by archiving email message and attachments for long-term storage
Digital Document Archiving powered by File Format Conversion
Convert any type of content into digital archive-ready format(s)
- Automatically prepare archive-ready content from your DMS & RIM & other desired locations
- Lightning fast conversion speeds. Scalable on Docker & Kubernetes
- Exceptional quality PDF & PDF/A conversion without manual intervention
- Handle all your content with 1 solution: MS Office, emails, images, audio & video, PDF and other file types
- Enhance the compliance of documents or content that goes into your digital archive
Trusted by Leading Life Sciences Organizations
For over 20 years, DocShifter has been trusted by pharmaceutical, biotechnology, animal health, and medical device companies around the world.
Built in close collaboration with regulatory teams, the platform continues to evolve to meet industry needs and stay aligned with changing regulatory requirements.
DocShifter is designed to streamline complex document conversion workflows and help your teams focus on what matters most.
Manage Complex Document Conversion with Ease
DocShifter is designed for the unique challenges of regulated industries. It supports a wide variety of document types used across clinical, regulatory, and quality functions.
Its high level of configurability and seamless integration with Ennov Doc, Ennov Dossier, Ennov Process, and other systems ensures it fits smoothly into your existing workflows.
Whether you’re preparing documents for eCTD submissions or internal reviews, DocShifter provides the flexibility and control you need.
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.