Ennov RIM -
Market Leading RIM Software
Multiple Vaults? Duplicate Data? Integrations between different systems? Limited configurability? High total cost of ownership?
Discover why Ennov RIM is the obvious choice
Gain control of your product details, registrations, submissions and regulatory activities
A purpose-built, 100% web-based Regulatory Information Management (RIM) system offering a single authoritative source for all regulatory data:centralizing product details, registrations, submissions, correspondence, and commitments. It streamlines regulatory operations and provides clarity, control, and responsiveness across global product portfolios.
 No integrations
No integrations
 No Silos
No Silos
 No Duplicated Data
No Duplicated Data
Here is why companies of all sizes trust Ennov RIM
- Scalable Portfolio Management
- AI-powered tools embedded in your workflows
- Fully XEVMPD & IDMP compliant
- End to end regulatory oversight
- One source of truth for documents, data, and audit trails
- Powerful analytics and insights
- 100% web-based, 21 CFR Part 11 compliant
- Streamlined operations and visibility
Your Single Authoritative Source of Regulatory Information
Imagine all of your regulatory information regarding products, registrations, submissions, correspondence, and commitments in one centralized place accessible from anywhere.
With Ennov RIM, life sciences companies can streamline their regulatory processes, improve their data quality, quickly answer business-critical questions and effectively respond to health authority requests.
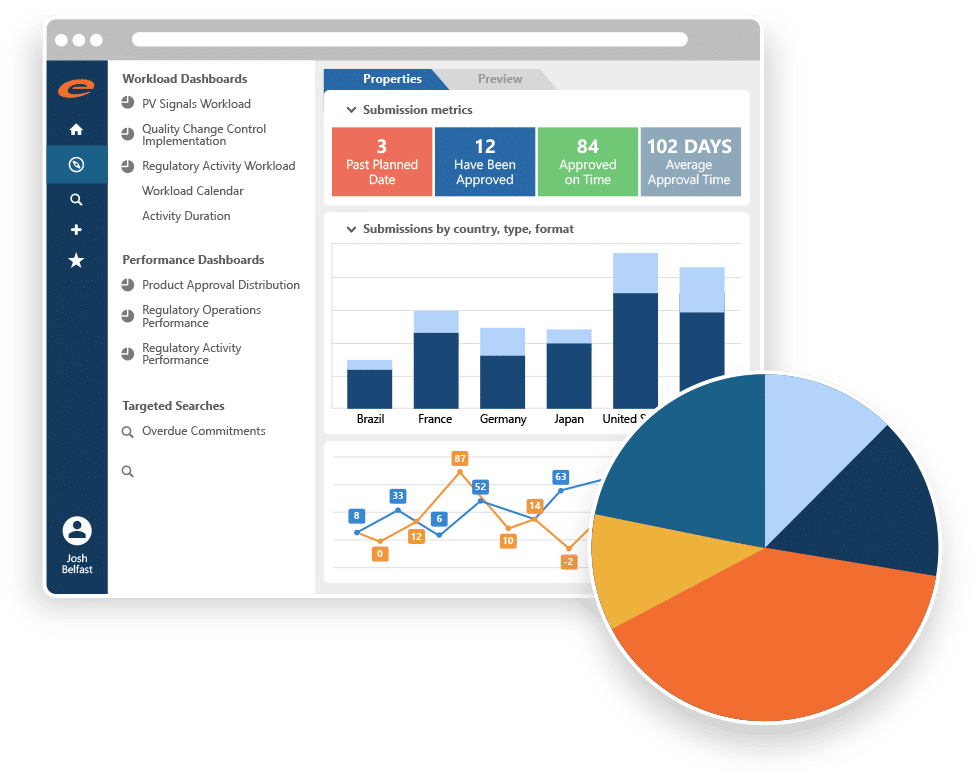
Core Capabilities
- Centralized management of detailed product information
- Market authorization and registration management
- Submission and Regulatory activity planning and tracking
- Automatic linkage between substances, products, registrations, activities, dossiers, submissions and documents
- Correspondence and commitment tracking
Key Features
- Role-based access and action rights
- Configurable data model
- Automated email notifications
- Intuitive user interface
- Robust workflow engine
- 21 CFR Part 11 compliant
Come join more than 450 Life Sciences companies around the world being powered by Ennov
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
Accelerate Your Regulatory Compliance
See how Ennov RIM streamlines dossier planning, submission tracking, and data visibility. Request your demo today.

World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.