Ennov QMS-
Market Leading Quality Management System
Multiple Vaults? Duplicate Data? Integrations between different systems? Limited configurability? High total cost of ownership?
Discover why Ennov QMS is the obvious choice
Take control of your GxP operations with an AI-powered QMS
Ennov Process allows you to automate your Quality processes and enforce the activities, participants and traceability required by your SOPs using our visual form builder and graphical workflow modeling utilities. Ad hoc processes or those that remain stalled for months are a thing of the past.
The “smart” data form associated with the workflow retains all of the information required at each process step and provides each participant with the information they need, in the proper context, to complete their tasks on time. Managers can monitor any workflow, visualize pending and completed tasks and intervene when tasks become delayed.
Automatic alerts, email notifications and escalations keep your processes on schedule and your business running smoothly.
 No integrations
No integrations
 No Silos
No Silos
 No Duplicated Data
No Duplicated Data
Here is why companies of all sizes trust Ennov QMS
- Pre-configured for common quality processes
- AI-powered tools embedded in your workflows
- "Smart" forms that adapt to process step and participant
- REST API for advanced integration
- One source of truth for documents, data, and audit trails
- Powerful analytics and insights
- 100% web-based, 21 CFR Part 11 compliant
- Automated email notifications
Ennov QMS supports a wide variety of Quality process management needs including CAPAs, Deviations, Non Conformances, Customer Complaints, Change Controls, Audits and more. Ennov QMS’ high degree of configurability and seamless integration with our Enterprise Document Management software (Ennov Doc), our innovative Learning Management software (Ennov Training) and our data visualization and reporting tools (Ennov Analytics) allows you the flexibility to meet your internal Quality standards as well as those of your business partners.
Easily Manage CAPAs, Deviations, Non Conformances, Complaints and more with Ennov QMS
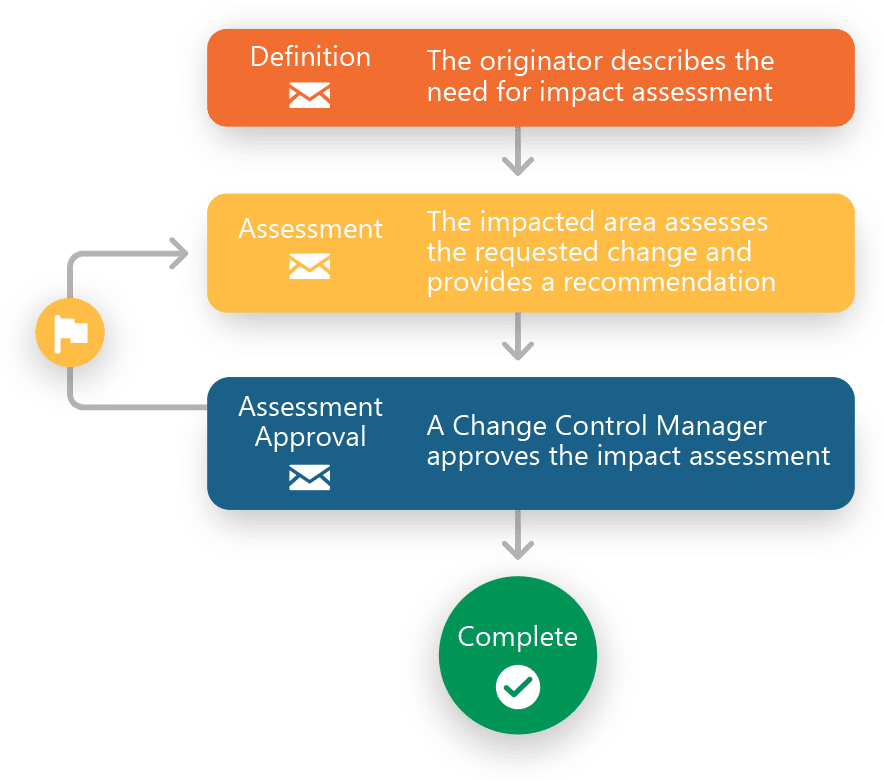
Core Capabilities
- Graphical workflow designer
- Powerful workflow engine that supports sub-processes
- Visual forms editor
- "Smart" forms that adapt to process step and participant
- Manual or automated task execution
- Real-time monitoring with dashboards and statistics
- Full text and metadata based searching
- REST API for advanced integration
Key Features
- Integrated work list dashboard
- Configurable processes, metadata and views
- Automated email notifications
- Intuitive user interface
- Pre-configured for common quality processes
- Intrinsically connected to Ennov Doc and Ennov Dossier
- 21 CFR Part 11 compliant
- 100% web-based
Come join more than 450 Life Sciences companies around the world being powered by Ennov
Pharmacosmos Case Study
“It makes our daily handling and signing of documents much faster, reduces the use of paper and eliminates signing documents by hand.”
Flemming Simonsen,
Director, GxP System Compliance Pharmacosmos
Unlock Your GxP Processes
See how Ennov QMS can keep you on schedule, and ensure your business runs smoothly

A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices.
This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.