Struggling to Squeeze IDMP Data Out of Your RIM?
Introducing the fastest way to get your regulatory data IDMP-ready—without overhauling your RIM.

What is EASI?
EASI Connector is Ennov’s Agnostic Solution for IDMP. This plug-and-play solution pulls structured, validated data from any RIM system and transforms it into IDMP submission-ready format.
The Ennov EASI Connector also connects to EMA’s Product Management Service (PMS), allowing you to compare your RIM data with PMS and prepare your enrichment for submission to EMA.
Why It Matters
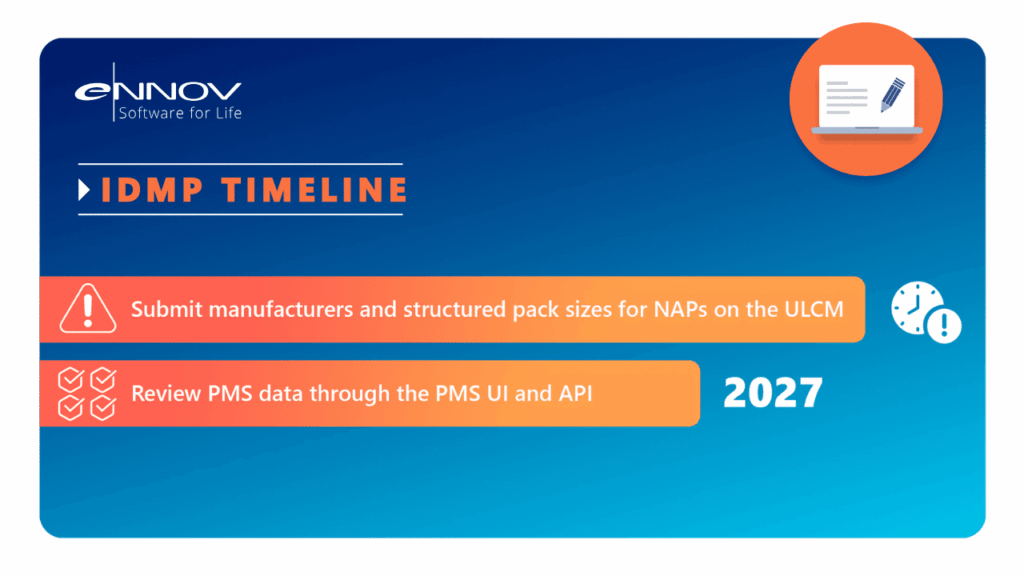
- IDMP Deadlines Are Closing In: EMA requires enrichment of PMS data. You can’t afford to wait.
- No Manual Rework: No data re-entry in the PLM portal. EASI automates the entire process.
- Stay in Control: Validate and compare your data with PMS before submission. Fix discrepancies once at the source.
How It Works
- Connects to Your Existing RIM: Agnostic integration—no matter your system.
- Extracts and Transforms Data: Seamlessly converts your product data into IDMP format.
- Fully Validated, Submission Ready: Fully aligned with EMA’s latest requirements. Future-proof and efficient.
Plan resourcing for IDMP PMS enrichment
Why EASI?
- Proven: Built by IDMP experts.
- Flexible: Works with any RIM or master data system.
- Fast: Submit IDMP data without disrupting your current workflows.
The Result
- Save Time
- Ensure Full SPOR Compliance
- Avoid Data Duplication
- Be Ready for PMS API Automation
- Reduce Manual Errors
- Partner with the Industry Leader in IDMP Compliance