Ennov RIM
Regulatory Information Management Software
Designed for Regulatory Affairs, Regulatory Operations, and registration teams, Ennov RIM centralizes product and registration data to support submissions and regulatory activities.
- Centralize product details, registrations, and regulatory data in one RIM system
- Connect regulatory data with documents and dossiers to reduce rework and duplication
- Automate regulatory workflows to keep activities on track and auditable
- Improve visibility with reporting and dashboards across products, markets, and timelines
- Support IDMP readiness by strengthening product data governance and consistency
The Regulatory Information Management (RIM) challenge
Regulatory teams are managing more products, more markets, and more change, often across spreadsheets, shared drives, and disconnected tools. That fragmentation creates inconsistent data, duplicate effort, and slower answers when teams need to understand registration status, submission plans, commitments, or upcoming lifecycle activities.
Ennov’s regulatory information management (RIM) software addresses this by centralizing product and registration data in a structured system of record. With consistent data and controlled processes, teams can reduce rework, improve visibility across markets, and respond faster to business-critical questions. It also strengthens data governance, which supports initiatives like IDMP readiness as requirements evolve.
Ennov RIM: a single source of truth for regulatory information
Imagine all of your regulatory information regarding products, registrations, submissions, correspondence and commitments in one centralized place accessible from anywhere. With Ennov RIM, life sciences companies can streamline their regulatory processes, improve their data quality, quickly answer business-critical questions and effectively respond to health authority requests.
Ennov RIM is regulatory information management software that centralizes product, registration, submission, correspondence, and commitment data in one place. With consistent data and shared visibility, regulatory teams can answer business-critical questions faster, respond to health authority requests with confidence, and reduce rework caused by fragmented tools.
Built for Regulatory Affairs and Regulatory Operations, Ennov’s RIM software supports global portfolio oversight, including launch planning, variations, and ongoing lifecycle activities. Structured data entry, role-based workflows, and reporting help teams track regulatory work across products and markets and keep execution on schedule.
Increase Operational Efficiency with Ennov RIM
Ennov RIM helps regulatory teams turn data into coordinated action by clarifying what needs to be done, when, and by whom. Workflow-driven processes, notifications, and real-time status updates improve visibility into assignments and deadlines, so work moves forward with clear ownership and fewer manual follow-ups.
Dashboards and reporting provide a portfolio-level view of regulatory activity across products and markets, helping teams prioritize work, identify bottlenecks, and keep registrations up to date. The result is a more predictable way to manage lifecycle activities and reduce the risk of missed tasks and avoidable delays.
Ennov RIM supports a broad range of product types, including pharmaceuticals and medical devices, with configurable structures to match your portfolio.
Fully Compliant XEVMPD Submissions
Ennov RIM includes automated, compliant and efficient submissions to XEVMPD. Messages are compiled, validated and submitted through Ennov RIM. Our integrated workflows ensure that XEVMPD compliance can be achieved and maintained with ease.
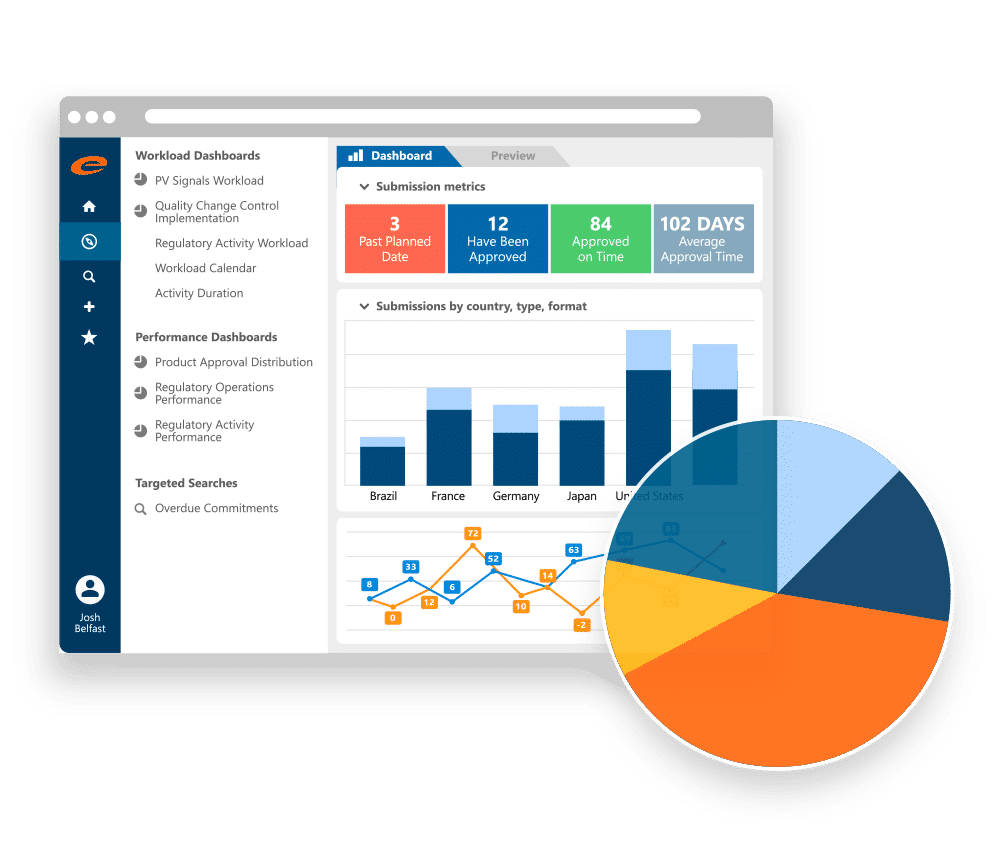
Dashboards for Planning, Impact Analysis, and Reporting
Ennov RIM dashboards give regulatory teams a clear view of workload, deadlines, and overdue activities across products and markets. Regulatory Operations can quickly see what is coming due, what is at risk, and where follow-up is needed, without pulling reports from multiple sources.
Teams can also run impact analysis to understand which registrations may be affected by a change, for example a manufacturer update or formulation change. Out-of-the-box dashboards cover key regulatory activities, including submissions, correspondence, and commitments, and help leadership track timeliness and volume over time.
Specialized dashboards are also available for use cases such as PSUR tracking and additional impact analysis views.
Core Capabilities
- Centralized management of detailed product information
- Market authorization and registration management
- Submission and Regulatory activity planning and tracking
- Automatic linkage between substances, products, registrations, activities, dossiers, submissions and documents
- Correspondence and commitment tracking
Key Features
- Role-based access and action rights
- Configurable data model
- Automated email notifications
- Intuitive user interface
- Robust workflow engine
- 21 CFR Part 11 compliant
RIM Software FAQs
What does Ennov RIM centralize in one system of record?
Ennov’s regulatory information management software (RIM software) centralizes regulatory information across product details, registrations, submissions, correspondence, and commitments. By keeping these elements connected in one place, teams reduce version conflicts and can answer common questions faster, for example, what is approved where, what is due next, and which activities are at risk.
How does RIM software help manage submissions and ongoing lifecycle work?
A RIM system is not only for tracking approvals, it supports execution. Ennov RIM software helps teams plan and manage regulatory activities tied to submissions and lifecycle events, including ownership, deadlines, and status, with workflow-driven steps and notifications. This reduces manual follow-up and improves consistency across products and markets.
How does Ennov RIM reduce duplicate data entry and inconsistent information?
Ennov regulatory information management software helps reduce duplication by linking related regulatory information, so updates can be managed consistently across products, countries, and applications. Instead of maintaining the same information in multiple spreadsheets and reports, teams work from a controlled source of truth with traceable updates.
What workflow and automation capabilities should a RIM software platform provide?
A modern RIM software platform should support configurable workflows that reflect SOPs, define who does what and when, and keep a record of actions and decisions. Ennov RIM supports workflow-driven process automation with task routing, notifications, and real-time status updates, so teams can keep regulatory work moving and avoid stalled activities.
What dashboards should you expect in regulatory information management software?
Regulatory information management software should provide dashboards that help teams manage workload and oversight, not just store data. Ennov RIM dashboards help Regulatory Operations monitor upcoming workload, due and overdue activities, and portfolio-level metrics, while enabling leadership to track volume and timeliness across regulatory activities.
What is impact analysis in a RIM system, and why does it matter?
Impact analysis helps teams understand how a change affects registrations across markets, for example, when there is a manufacturer update or a change related to an active substance or excipient. In Ennov’s RIM system, impact analysis supports faster decision-making by showing which registrations may be affected, helping teams plan the right regulatory actions with fewer manual checks.
How does RIM software support IDMP readiness without replacing your IDMP program?
RIM and IDMP both rely on consistent, governed product data. Ennov RIM software supports IDMP readiness by strengthening product data governance, improving consistency, and connecting regulatory data with downstream activities. For organizations that need dedicated IDMP enrichment or alignment workflows, Ennov also offers IDMP-focused resources and solutions.
What should a regulatory information management system support for correspondence and commitments?
A practical regulatory information management system should help teams track correspondence and commitments with clear ownership, deadlines, and status, so nothing gets lost across email threads. Ennov RIM connects these items to products and registrations, helping teams maintain continuity and oversight across ongoing regulatory work.
Discover Our RIM Resources

Podcast - eCTD 4.0 Pilots: Cleared for Takeoff!
[White Paper]
The Impact of Ennov RIM at Foghorn Therapeutics
Efficient regulatory information management can be complex. Your software shouldn’t be.
Aguettant Case Study
“It’s really nice to work with a software provider that shows a great deal of innovative spirit, has great ambition and is proactive. Above all, I really appreciate Ennov placing particular emphasis on the customer’s user experience.”
Cyrille Jeune,
Regulatory Affairs Systems Manager

Septodont Case Study
“With more than 1500 MAAs in 150 countries, we face a real productivity challenge. With Ennov, we have been able to issue 400 dossier in just 18 months. For the first time, our users are experiencing tremendous time savings when locating documents.”
Aurélie Becquet,
Regulatory Affairs
World-Class Regulatory Content and Information Management
A Regulatory suite with the power and flexibility to support the entire regulatory product lifecycle from the early planning of registration targets through to product retirement.
It is an invaluable solution for regulatory activity planning, product registration management, dossier creation, dossier management and more.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.
Cloud-based or On Premises
Multi-Platform
ISO 9001 & 27001 Certified
How can we help you?
Fill out the form and we'll be in touch.