We are pleased to inform of the release of version 8 of the Ennov Platform!
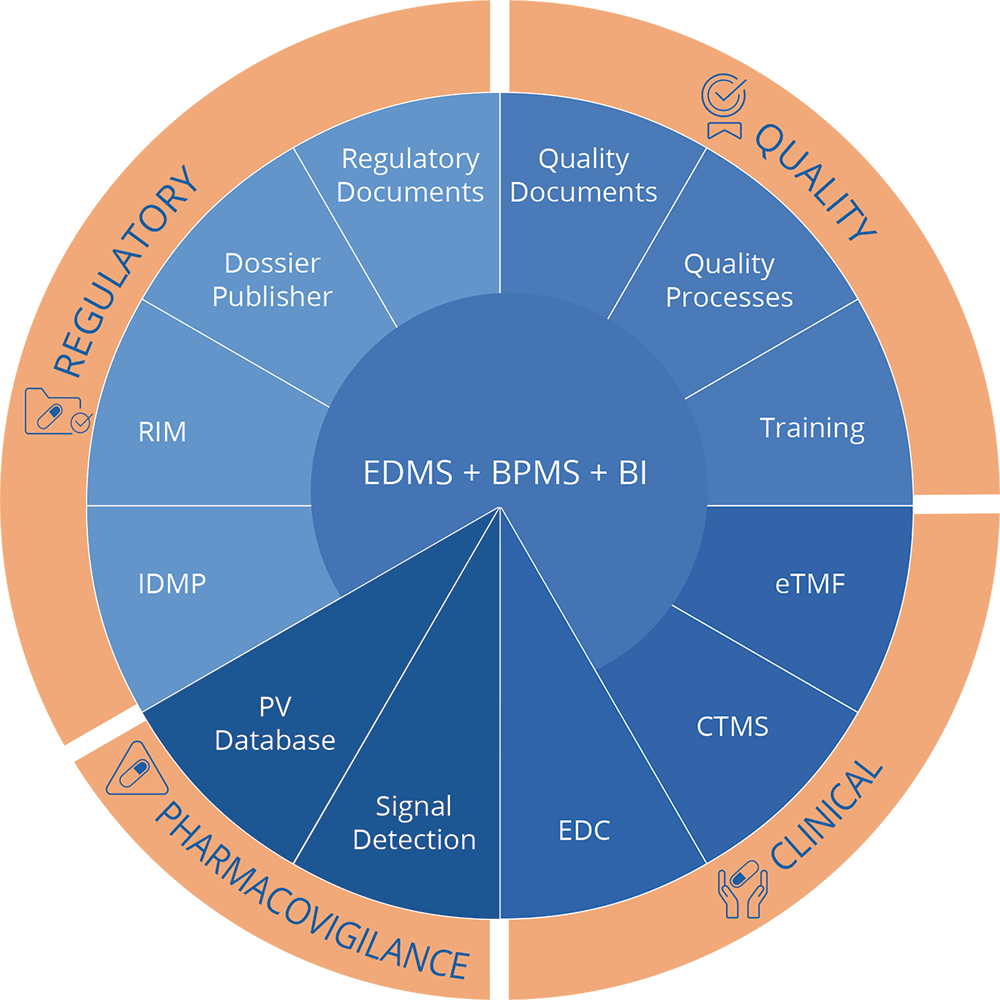
Based on a modern EDMS and BPMS, the Ennov Platform enables you to manage Documents, Workflows, and Data in a unified way. The Ennov platform is the technological base for Ennov’s Regulatory and Quality applications, as well as Ennov’s eTMF and CTMS. Our platform based applications such as QMS, RIM and submission publishing, are also natively integrated.
All these applications benefit from the platform updates including the modern and intuitive interface, Office 365 and Google Drive integration, and easy metadata navigation.
The Ennov platform is perfectly suited for Life Science (21 CFR part 11 compliant), and open to integrations through its REST API.
Learn more about Ennov 8 and its new features.
The New Ennov 8 is Now Available!
September 18, 2017
About Ennov
Ennov provides a comprehensive software platform to manage the most demanding processes of life sciences organizations in a compliant and efficient way. With over 25 years of experience, Ennov’s cloud-based solutions cover Regulatory Affairs, Pharmacovigilance, Quality, Clinical, and Document Management. Dedicated to innovation and excellence, Ennov’s solutions are used by more than 450 Life Science companies and 500,000 users worldwide, helping them to bring their products to market faster while maintaining compliance with regulatory requirements.
Share :
Facebook
Twitter
LinkedIn

Register for free to access to our library of exclusive content!

- White Papers
- Case Studies
- Product Demos
- Industry Briefs
- and more...

