QMS Software for Quality Management & Compliance
Designed for Quality, Regulatory and Operations, Ennov’s Quality management software digitizes and standardizes processes, while maintaining inspection readiness across regulated life sciences teams.
- Centralize quality processes and records in one QMS system
- Strengthen compliance with controlled workflows, traceability, and audit-ready documentation
- Improve visibility across deviations, CAPAs, change control, complaints, and audits
- Reduce manual effort with automated assignments, approvals, and reminders
- Scale quality operations across sites, products, and teams as you grow
- Understand your data thanks to pre-configured views, reports and dashboards
Quality management system software for regulated teams by Ennov
QMS software helps regulated organizations run quality processes in a consistent, traceable way while reducing manual effort. Instead of managing critical activities across paper, spreadsheets, and disconnected tools, quality management system software centralizes workflows, records, and approvals so teams can maintain inspection readiness and improve day-to-day execution.
Ennov QMS supports a fully-digital, paperless QMS approach by digitizing key quality processes, strengthening governance, and providing clear visibility into ownership, status, and deadlines. The result is a more standardized way to manage quality across sites and teams, with the controls needed for regulated environments.
Streamline quality processes with QMS software
Ennov QMS helps quality teams automate and standardize core QMS workflows, so activities follow your SOPs and every step is documented with the right level of traceability. Instead of relying on email and manual tracking, teams can manage structured processes in one place with clear ownership, status, and due dates.
Using configurable forms and workflow steps, each task captures the information required at that stage and routes it to the right reviewers and approvers. Managers can monitor progress in real time, see what is pending or overdue, and step in quickly when a process is delayed.
Automated alerts, notifications, and escalations help keep quality processes on schedule and reduce delays caused by manual follow-up.
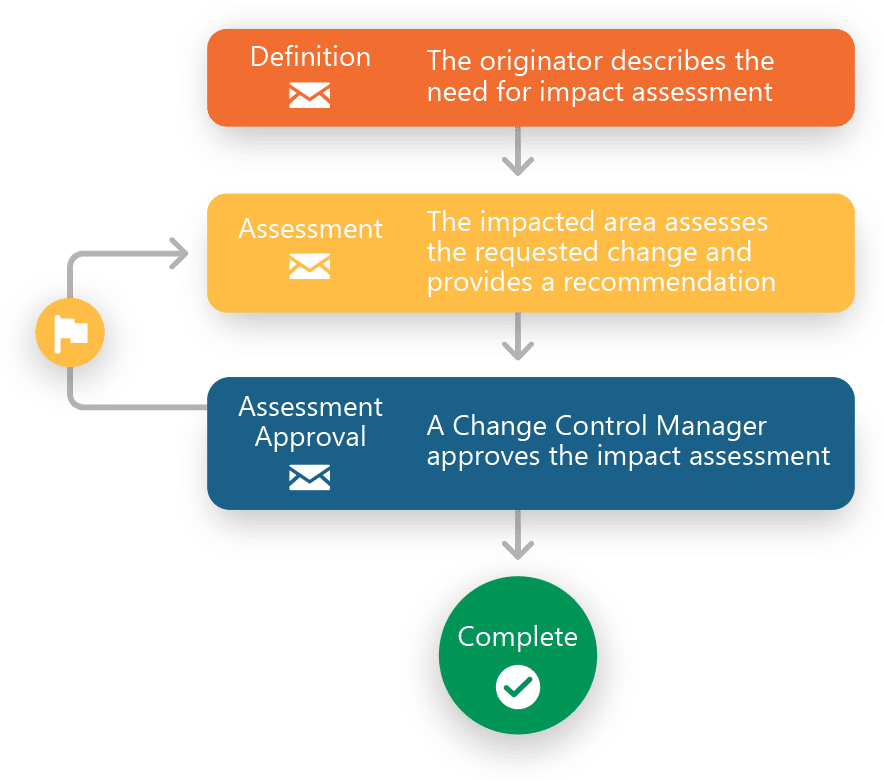
Manage CAPAs, Deviations, Non Conformances, Complaints and more with Ennov QMS
Our customers use Ennov QMS to support a wide variety of Quality process management needs including CAPAs, Deviations, Non Conformances, Customer Complaints, Change Controls, Audits and more. Ennov QMS’ high degree of configurability and seamless integration with our Enterprise Document Management software (Ennov Doc), our innovative Learning Management software (Ennov Training) and our data visualization and reporting tools (Ennov Analytics) allows them the flexibility to meet their internal Quality standards as well as those of their business partners.
As an added benefit, Ennov QMS fully complies with FDA’s 21 CFR part 11 requirements (electronic signature, audit trail, records management), making it a perfect fit for regulated industries such as pharmaceutical, biotechnology, animal health, medical device and others.
Learn more about :
Go Live Faster With the Preconfigured Ennov QMS Core Model
Ennov QMS includes a preconfigured core model based on common quality management best practices, so teams do not have to start from a blank slate. It comes with ready-to-use workflows for core QMS processes, then you can configure fields, roles, and approval paths to match your SOPs and terminology. This approach reduces setup time, supports a more consistent rollout across sites, and helps organizations go live faster while keeping governance and traceability in place.
- DEVIATION
- CHANGE CONTROL
- COMPLAINT
- CAPA
- IMPACT ASSESSMENT
- AUDIT FINDING
- AUDIT PROGRAM
- PRODUCT RECALL
- OPERATION TASK
Core Capabilities
- Graphical workflow designer
- Powerful workflow engine that supports sub-processes
- Visual forms editor
- "Smart" forms that adapt to process step and participant
- Manual or automated task execution
- Real-time monitoring with dashboards and statistics
- Full text and metadata based searching
- REST API for advanced integration
Key Features
- Integrated work list dashboard
- Configurable processes, metadata and views
- Automated email notifications
- Intuitive user interface
- Pre-configured for common quality processes
- Intrinsically connected to Ennov Doc and Ennov Dossier
- 21 CFR Part 11 compliant
- 100% web-based
QMS Software FAQs
What is Quality Management System (QMS) Software?
QMS software is a digital system used to manage quality processes, records, and approvals in a consistent, traceable way. It helps regulated organizations replace manual tracking (paper, spreadsheets, email) with controlled workflows, standardized data capture, and a clear audit trail. The result is better visibility, stronger accountability, and faster execution of quality activities without sacrificing compliance.
What is quality management system software used for?
Quality management system software is used to standardize how quality work gets done across teams and sites, and to centralize the records that support compliance. Typical use cases include managing deviations, CAPA, change control, audits, complaints, training-related workflows, and document-controlled processes, depending on scope. It also supports reporting and oversight by making it easier to track status, ownership, cycle times, and recurring issues.
What processes does Ennov QMS support out of the box?
Ennov QMS includes a preconfigured set of core quality workflows so teams can start with proven process foundations instead of building from scratch. Out of the box, the model includes workflows for Deviations, CAPA, Change Control, Complaints, Audit Program, Audit Findings, Impact Assessment, Product Recall, and Operational Tasks. These workflows can then be configured to match your SOPs, terminology, roles, and approval paths.
What is the Ennov Quality Core Model, and what are the benefits?
The Ennov Quality Core Model is a preconfigured framework that provides ready-to-use quality processes, records, and master data structures based on common quality management best practices, and 25+ years of experience. It helps teams go live faster by providing a consistent starting point for workflows and governance, reducing setup effort and rework during rollout. It also supports a more standardized deployment across sites and teams, while keeping traceability and controlled updates in place.
What is the difference between a QMS system and QMS system software?
A QMS system is the full quality framework, your processes, SOPs, governance, roles, and responsibilities. QMS system software is the technology layer that helps you execute that framework consistently by enforcing steps, routing approvals, controlling updates, and capturing the required evidence. In practice, software reduces variation between sites, improves traceability, and makes it easier to maintain inspection readiness as your organization scales.
What should I look for in QMS compliance software for regulated life sciences?
In regulated life sciences, QMS compliance software should support controlled workflows, role-based access, and reliable traceability for every step and decision. Look for strong audit trails, consistent document and record control, and the ability to standardize processes across sites while still allowing configuration to match your SOPs. It should also support operational oversight through dashboards and reporting, so teams can monitor risk, bottlenecks, and recurring quality events.
What is paperless QMS software?
Paperless QMS software digitizes quality processes and records so teams can manage QMS execution without relying on paper forms or manual follow-up. Instead of fragmented documentation, workflows capture required information at the right step, route tasks to the right owners, and keep a complete history of updates and approvals. This improves consistency, reduces delays caused by manual coordination, and helps teams stay inspection-ready with faster retrieval and clearer traceability.
Can QMS software support pharma and biotech quality management?
Yes. QMS software for pharma and biotech supports regulated quality workflows by standardizing execution, strengthening governance, and improving visibility across sites and stakeholders. It helps teams manage high volumes of quality events, maintain controlled records, and track corrective actions and effectiveness over time. It also supports scaling, for example, when adding new sites, products, partners, or operating models, while keeping processes consistent and audit-ready.
Discover Our QMS Resources

Keep CMC changes audit-ready
[Read the Article]

A blueprint for scalable QMS
[Practical Guide]

Design, scale, and validate with confidence
[White Paper]

How Vetoquinol unified global compliance
[Blog Article]

AI-powered training compliance
[Product Demo]
Come join more than 450 Life Sciences companies around the world being powered by Ennov

Delpharm Standardizes Quality Across 16 Sites…
with a paperless, audit-ready quality ecosystem that scales with growth and accelerates new site integration.




Turning Quality into a Growth Engine



Pharmacosmos Case Study
“It makes our daily handling and signing of documents much faster, reduces the use of paper and eliminates signing documents by hand.”
Flemming Simonsen,
Director, GxP System Compliance Pharmacosmos
A Comprehensive QMS to Improve Efficiency and Ensure Compliance
Ennov Quality provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best practices.
This allows your organization to get your QMS system into production and begin realizing your return on investment more quickly. Ennov Quality, like all Ennov solutions, is easy to configure and requires no IT skills.
Why Choose Ennov
Over 500,000 users trust Ennov
Over 500,000 users trust Ennov
- Over 25 years of experience providing software solutions for Life Sciences and 450+ Life Science customers, with many more in other industries.
- Modern architecture and interface 100% web-based. Highly scalable. User-centric design.
- Our commitment to your success Very high customer satisfaction, 98.5% of projects delivered on time and within budget.
Providing you freedom of choice
Providing you freedom of choice
- Available as cloud-based or on-premises deployment, you can switch between deployment options at any time.
- System configuration and management require no IT skills, making you fully autonomous
- Improved security and optimized performance. Data can be hosted locally for total flexibility. Single-tenancy minimizes business interruptions.

